My answer was
3HCOOH+2HCHO+CO2 bt the actual answer is 4HCOOH+2HCHO!!! How is it coming??????
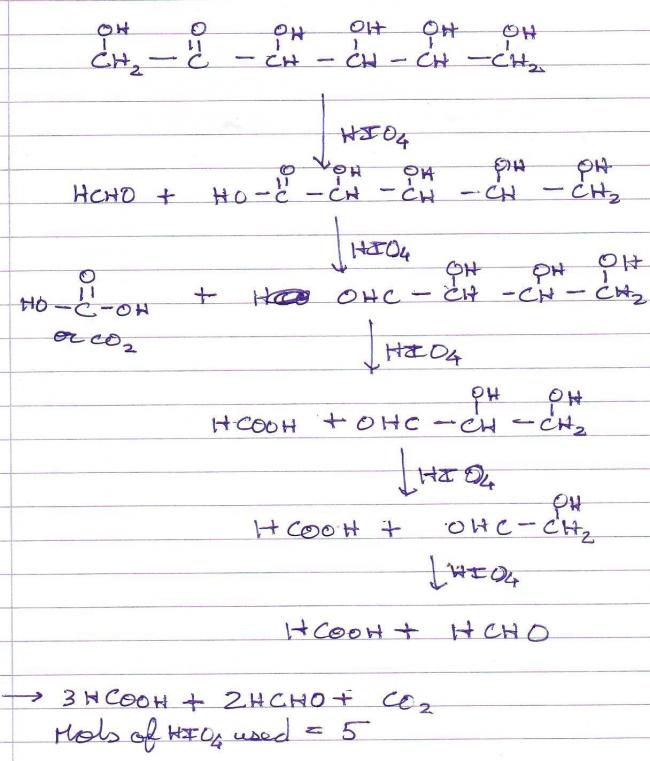
CH2OH-CO-CHOH-CHOH-CHOH-CH2OH +HIO4→ ????
( α D-Fructose)
1ST OF ALL...ISNT 5 MOLE HIO4 NEEDED???
2ndly Predict the products.........plzzzz help!! :(
This might be helpful for you
In case of simple polyhydroxy compounds, for every two adjacent hydroxyl groups, one
mole of HIO4 is used. Therefore, if there are 'n' adjacent hydroxyl groups, ( n-1) moles of
HIO4 would be used. Thus by knowing the number of moles of HIO4 used, the number of
adjacent hydroxyl groups in a molecule can be determined. For example, oxidation of
glycerol uses two moles of HIO4 per mole of glycerol. therefore, it contains three OH
groups.
HOCH2-CHOH-CH2OH + 2 HIO4 .......> HCHO + HCOOH + HCHO
It may be noted that the internal OH groups are oxidized to formic acid during oxidation.
However, in case of polyhydroxy aldehydes or ketones, since oxidation of -CHOH-CHO or -
CH-CH2OH moity requires one mole of HIO4, therefore, number of moles HIO4 used is
equal to number of adjacent OH groups present. For example, oxidation of one mole of
glucose requires five moles of HIO4.
HOCH2-(CHOH)4-CHO + 5 HIO4 ........> 5 HCOOH + HCHO
HOCH2-(CHOH)3-CO-CH2OH + 5 HIO4 ......> 2 HCHO + 3 HCOOH + CO2
From the above discussion, we can say that CO group is oxidised to CO2, CH2OH to HCHO
and CHO,CHOH to HCOOH.
In the given problem three moles of HIO4 are used and the products are two moles of
formic acid , one mole of formaldehyde and one mole of CO2; thus the structure should be
CHO-CHOH-CO-CH2OH
My answer was
3HCOOH+2HCHO+CO2 bt the actual answer is 4HCOOH+2HCHO!!! How is it coming??????
Nice joke.
Pritish and Qwerty are my mentors in organic and they help me out everytime, so I can never become better than them and there concepts are just awesome, crystal clear. Even If I study day and night organic I wont be able to match up with Pritish at any cost.
@ Abhishek tumhari book ka answer wrong hai, even Pritish is getting the same answer as you and me
Bas Khyati..bas :P
Firstly, I'd recommend that you check out page number 29 of http://www.scribd.com/doc/30469233/Alcohols-Phenols-Ethers-My-Notes if you're not clear with the periodic acid oxidation of alcohols.
Secondly, here's me solution(can't guarantee that it's correct) -:
I too think its the correct answer.....hope so!! thanks for the help though.....khyati and pritish bhaiyya! :)