can u show the elec. config. and hence put your point
which is more stable carbene......singlet or triplet....explain with reason!!!
-
UP 0 DOWN 0 0 12

12 Answers
triplet more stable as it has symeetrical charge distributn nd min repulsion
http://en.wikipedia.org/wiki/Carbene
Triplet is the more stable state (the ground state) and singlet is the excited state species. Substituents that can donate electron pairs may stabilize the singlet state by delocalizing the pair into an empty p-orbital. If the energy of the singlet state is sufficiently reduced it will actually become the ground state. Triplet carbenes are generally stable in the gaseous state, while singlet carbenes occur more often in aqueous media.Triplet carbenes can be considered to be diradicals, and participate in stepwise radical additions.It also has symetrical charge distribution.

Figure from wiki and notes from morrison
is this the electronic structure???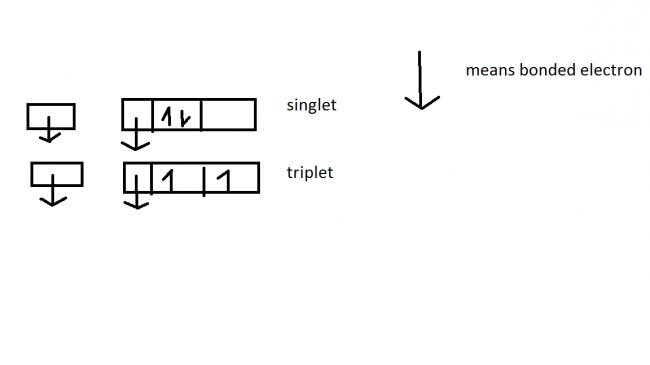
now comment on stability via their elec. config.
@tanay....thanks!!! i saw the link but i wanted to confirm with the elec. structure
yups a triplet is more stable coz it follows hund's rule....
and we can see it also like this.......a singlet is formed when a triplet's electron is excited...by giving some energy...so naturally the singlet has to be unstable
triplet is a more stable carbene den a singlet ... in d structures v can clearly see
as singlet has 2 bond pairs and 1 lone pair n 0 unpaired electron..
and triplet has 2 bond pair and 0 lone pairs n 2 unpaired electron..
whnevr in a reaction if singlet carbene is formed it remains unreacted n gets converted to more stable form of a triplet ..
also ... it follows hunds rule... in triplet case but doesnt follow in d case of singlet carbene
tu mil ab grandmaster... sab ko gumrah kar raha hai jee ke odin pahle... carbene nahi poochta JEE me