briliant work srinath....
i am sorry to confess that it was my self-made question...
hope it was not a time waste solving this...
thanx srinath n nishant as well....
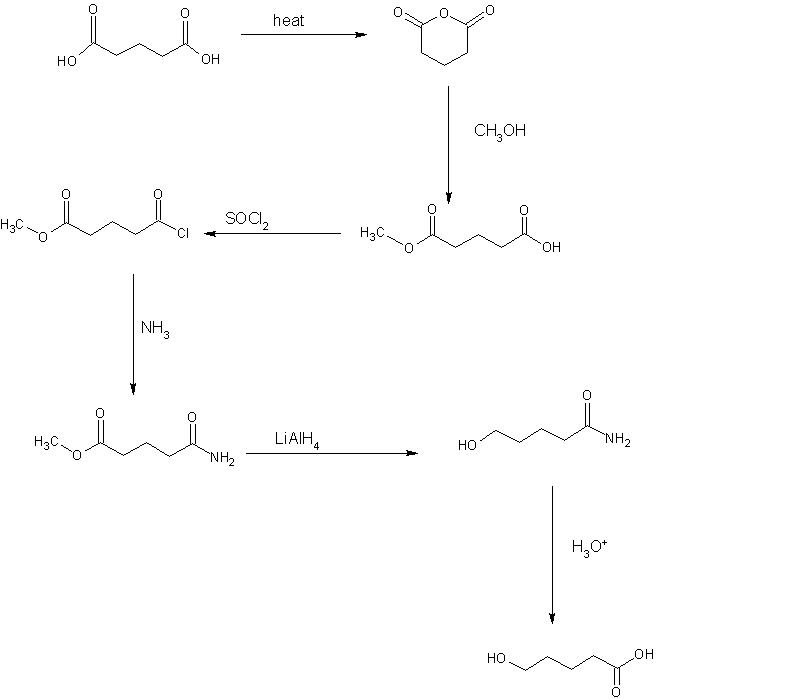
can nyone tell me........... if there are two COOH groups in one molecule, then how to change one to CH2OH keeping another intact??
plz help
-
UP 0 DOWN 0 0 18

18 Answers
Sky , I'm sorry . The method is wrong.
THere's a slight problem.
I'll post the correct soln in another day .
LAH reduces even the amide . I'll just take diversion there possibly.
tll then it's fine.
Possibly . we can then , carry out Hofmann's degradation and then add NaNO2 with HCl . this will give a comp containing ester gp and also a salt. I forget what it's called . It has a N2+ and Cl- . then add water to get teh desired product. I think.
I'll confirm in a day.
Skygirl , why should you feel sorry ? I should say ,thank you . It's brilliant.
It'll help us prepare better . And this definitely isn't a waste of time. It covers a good lot of concepts.
Arjita , why do you think CH3+ attacks? It's actually CH3O- which attaks . Remember that there are electrophilic carbons present in the substrate. not nucleophilic.
Abhishek , your method is fine , yet in any given organic reacn. the yield is of utmost importance. there are possibility of two SOCl2 molecules reacting with the same molecule.
Also , Rosendmund redn , is very unreliable as a practical reacn as it yields abt 8-9 byproducts which are difficult to separate, even if you do it , there is always a chcnce of getting impure products.
You're essentials are correct , otherwise .
but a lot of assumptions.
Read 5000 Named Reactions.
available on the net.
One more method..........(3 steps only hopefully it will work)
Step 1add 1 equivalent of SOCl2 since molecule has symmetric -COOH groups therefore it does not matter which -COOH is converted to -COCl
Step2now perform Rosenmund reduction(H2 Pd BaSO4) to convert -COCl to -CHO leaving -COOH as it is.
Step3Now add NaBH4 it will reduce -CHO to -CH2OH leaving -COOH undisturbed.
Ok if U r not happy with that step of conversion frm ether 2 alcohol use HBr in presence of aq H+ then Oxygen will protonated and Br- will attach -CH3 by SN2 and make CH3Br and the desired alcohol.
i had one doubt in the soln... how u splitted CH3OH IN ch3+ on OH- although oh- is a poor leaving group plzz tell
Please give the molecule . then It'd would be much easier. because . It depends on structure I think.
i think u could try making an ester.. then break it such that there are 2 different functional groups.. amine kind?
so then u could proceeed to do what u want.. just a guess.. i am not good at organic.. but just have been seeing this unsolved for long now!
try remving one cooh group and then do your work with the other cooh group ....and try placing a cooh group again ....its just an idea....
yaar.... watever i am trying to do is happening with both of them!!! i dun think this is possible.....
I don't think it's possible to reduce only one selectively . A many step process may be possible.