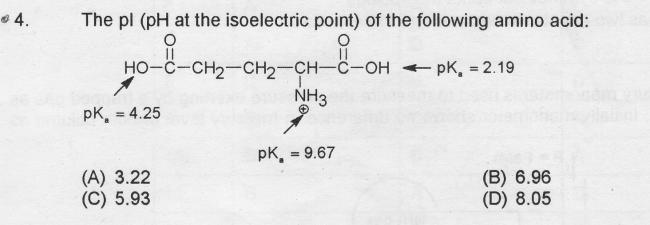
At the isoelectric point, the molecule shouldn't have a net charge. In that case, as NH3 is already positively charged, we wish to have one of the H - from two carboxylics to be removed.
The pH for that should be the mean of the two Pka's of the COOH groups.
i.e, pI = 3.22