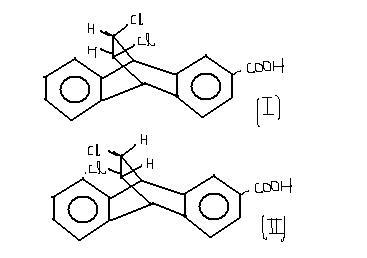
just a guess.. dunoo whether i am right or wrong
i think that II is more acidic than I bcoz after losing the H+, carbon ion will be formed which will be resonance stabilised by Cl and moreover the π electron cloud of the benzene ring(the one on which -COOH) is attached will stabilise the carbon ion in structure II more effectively as compared to structure I.
5 Answers
Which one do you think is most acidic H?
I think COOH
btw pKa of 1 = 5.67 and II is 6.07
so 1 is more acidic
ya dude..sry what answer i gave was abt pKa2 ....
actually 1 is more acidic than 2 ..due to a phenomenon called Field Effect...it is just the same like Inductive effect..but in this case the more elcronegative element is not directly binded tho the grp but still it withdraws the electron density from it making it more acidic..
So in case I consider Cl and the benzene ring containing the COOH grp in 1 plane..so Cl withdraws the electron density from the ring making it more acidic..while in Case II Cl is on the other side...
no yaar .. i dont think that JEE will ask such kinda question....
by the way Ashish check this one
http://targetiit.com/iit-jee-forum/posts/optical-activity-14234.html
looks like there is some problem in the question...
it shud be cyclohexane..