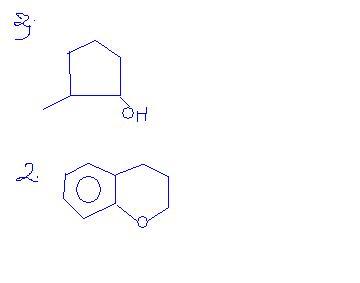
in Q2, along with Clemmensen Reduction, HCl can act up oxirane and cleave it to form one hydroxy and chlorine group, but it is not favorable and the reaction will reverse back
4 Answers
RDX
·2009-09-23 10:12:07
RDX
·2009-09-23 10:13:53
IN Question 1, if H2 supply is limited then it will cleave the very first double bond from left.. because right one double bonds are stable by conjugation and will remain untouched until high temperature is supplied to break the pie bonds and more energy to break d conjugation point.
Anyone with different view toward it, please answer
Debotosh..
·2009-09-23 10:18:17
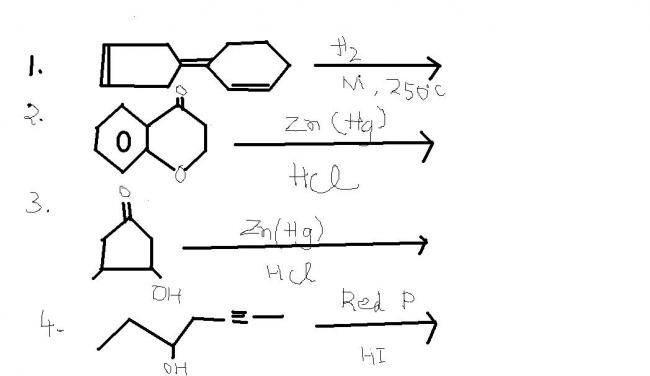
ans 3 ----- 2 methyl chloro cyclopentane
the substrate is acid sensitive ...hence this is the product !