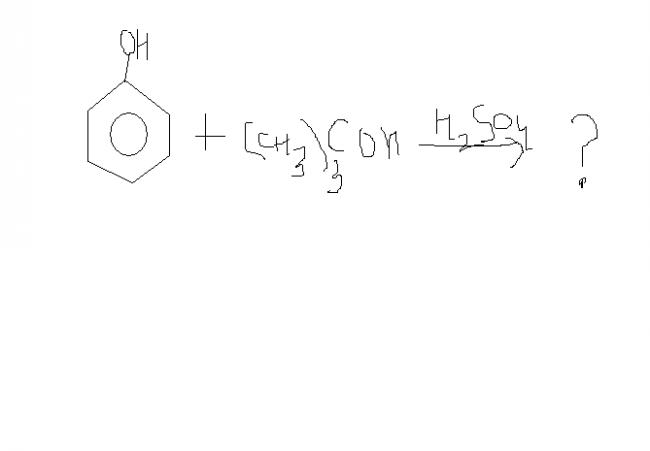There will be an -O also in the new added group at the joining position
3 Answers
Asish Mahapatra
·2009-09-20 06:49:20
(CH3)3COH + H+ --> (CH3)3C+
This electrophile will attack the phenol.
As phenol has o-p directing grp,
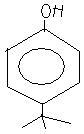
ortho as well as para product will form with para as major product because of steric hindrance
Kartik Sondhi
·2009-11-22 07:58:00
Pritish Chakraborty
·2009-11-22 08:31:25
A type of FC alkylation....OH will be protonated and leave, and the tert butyl carbocation will attack phenol at para position. Asish is correct.