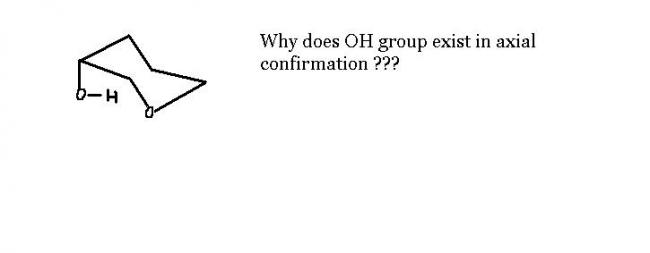1
1actually, when u compare just two conformations , one hving an OH group at equitorial and other at axial,then surely one hving at equitorial OH is more stable as more room is availabe at equitorial position than axial one.thus,more bulky groups at equitorial place , more is the confomation stable thn the other.
but,normally , groups like OH r linked with reactions like SN2 and E2 , thus these reactions wants OH to be present at the axial position fr frequently occuring anti-reactions.if not also anti reaction, the reactions are more progressable at axial positons as this position makes the compound a bit more reactive thn equitorial conformer.
 24
24but wouldnt a compound want stablity rather than undergoing reacitons ????
so it should be at equitoirial confirmation for stablity of compound ???
 1
1ya,it should be. but what if the reaction requires so.
for e.g. E2 reaction, frm the two conformations in which one has d leaving group at equitorial plane and other at axial plane.then,equitorial conformer is more stable,but the axial conformer undergoes this reaction as the reaction is anti. thus,it depends on requirement of reaction.
but,one more thing , if its possible dat more stable conformer forms the product then the rate of reaction as well as the yeild is increased