7 Answers
Kartik Sondhi
·2009-11-13 21:03:12
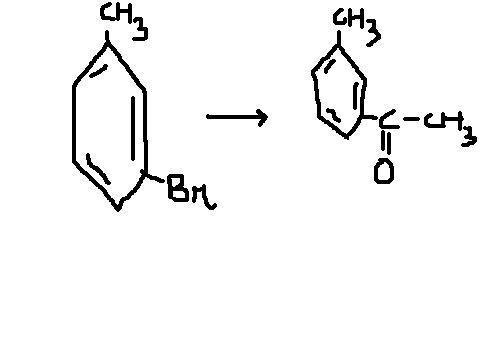
Simply by adding a stronger oxidzing agent than -Br that if -Cl or simply CH3COCl that is we can add with ether this will bring it in one Step
Pritish Chakraborty
·2009-11-16 06:21:57
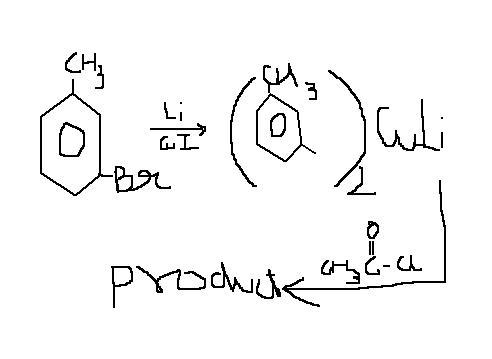
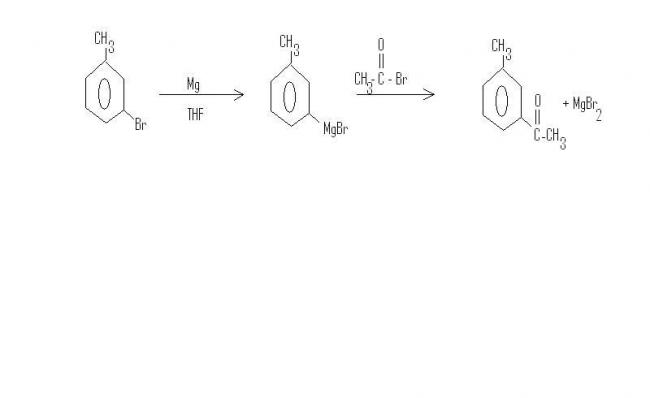
Nahi, I checked, Grignard reagent formation is a very useful route for these purposes. Problem tab hoti hai jab koi substituent ko hatana chaahe via substitution. There is no problem in addition.
The last step works out because magnesium bromide ka ppt banta hai, which shifts the equilibrium of formation far forward.