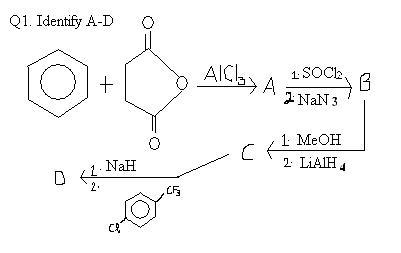
Ans 3 A..see when NaoH is added buffer is formed at point A ..then at point B first equivalence..then at C again buffer is formed then second equivalence at D
8 Answers
Q2) since pressure is low so pressure correction n2aV2 can be neglected.
So equation becomes, P(V - nb) = nRT Dividing by nRT througout we get,
PVnRT - PnbnRT = 1,
Z - bRTp = 1,
Z = 1 + bRTp ................which is option(b)
(not sure of the ans.)
i think since pressure is low, then volume is high.. so volume correction will be neglected...
??
nicely explained...
i was thinking all the time it was monobasic acid....
yes agree with Asish volume correction shud be neglected wen volume is very high
@Manmay we say 1 + α ≈ 1 wen α < < 1 ..so since volume is high so an2/V2 will be low and presuure is also given very less..so we cant neglect it..
then what should be the answer govind??
btw solved the first one?
ya. sorry i did the opposite thing.
So by neglecting Volume correction I guess ans will be (d)