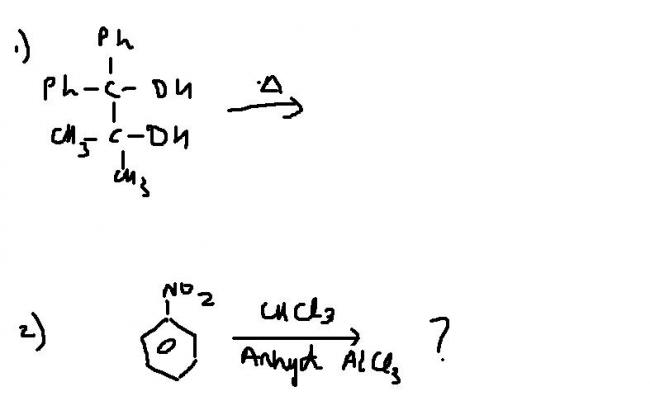1st reaction looks like pinacol rearrangement...diol and heat.
I was thinking in 2nd dichlorocarbene would form but it needs a base, not a lewis acid to alpha eliminate. Isn't it just friedel craft's reaction in low yield?
9 Answers
its pinacol...but isnt it some speical case ???becoz Ph wont migrate. ???
carbocation will be highly stable due to 2 Ph groups....
and how can 2nd be FCA ??
Bhai migratory aptitude wise phenyl group is ahead of methyl group, as far as I remember. Phenyl group will migrate best.
What else will 2nd reaction be, you reckon? I can't think much right now..am sleepy. lol
nitrobenzene does not respond to F.C. Reaction ! this is something else ! [12]
first one is pinacol stuff...carbocation at the site which has 2 Ph groups and then the usual stuff !!
The migratory aptitude is :-
Ph - > H - > R - (3° > 2° > 1°)
Electron rich group usually migrate in preference
Ans 1) 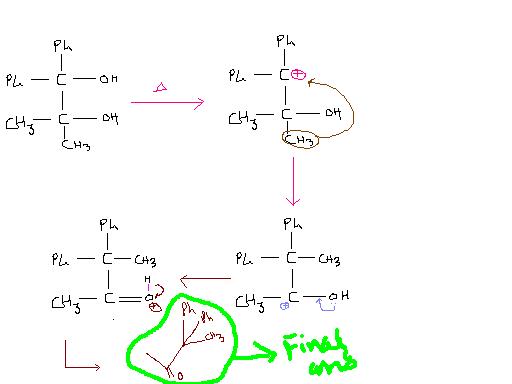
Edit : Okay got it...carbocation is more stable with two Ph groups attached. Migration would occur from the other end.
thats what i said,,,,thx tush for confirming
and prtish..2nd isnt FCA..its chloroform reacting ...any product ??