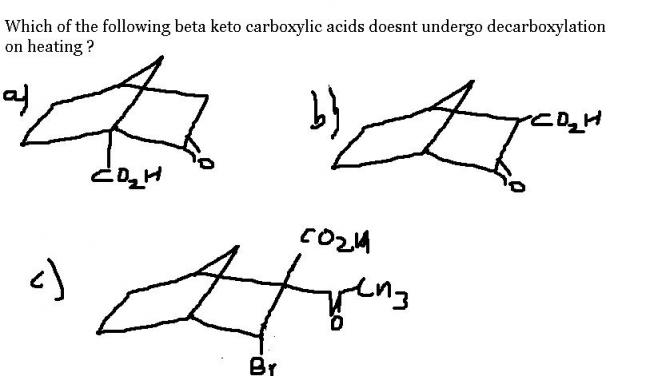
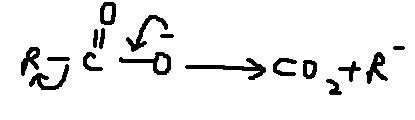but in decrboxylation reaction carbanion is formed naa ???
17 Answers
thx..organic for confirmation...
maybe aieeee made just that sign mistake...
yes to all who say that carbanion is the intermediate ! bridgehead carbanion is the most unstable in this context...hence (a) never undergoes decarboxylation !
@msp...you should not have pinked that post without confirmation...gives others a wrong concept here !
well eure thermolysis is a way to produce free radicals,i confirmed it in the peter sykes,(free radical chapter).
eure remove the + sign and put a dot in that place,nothing more.and we know the free radical is sp2 hybridised as like a carbocation.
actually i think free radical will be formed, and not carbocation formation.Neways the free radical formed shud be sp2hybridised,and it shud have a planar structure,so the intermediate is extremely unstable.
if decarboxylation occurs in a) , then -CO2 would be eliminated forming bridgehead carbanion as transition intermediate , which is very unstable.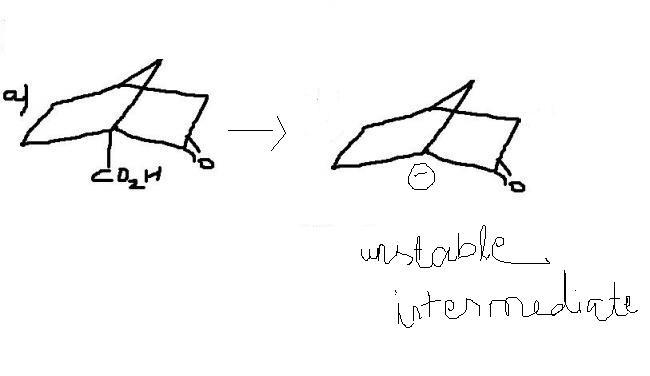
thus answer is a.