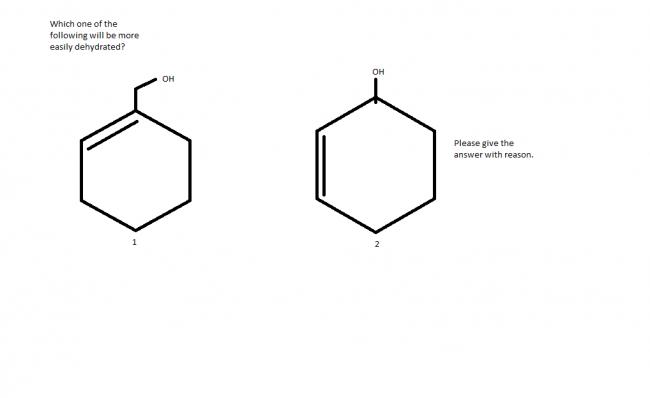
obiviously second one because 1st one forms 1° carbocation as intermediate and allylic where as second one is 2° carbocation and allylic
12 Answers
On dehydration, both of them give rise to planar allyl resonance stabilized cations...but my hunch is that in the 2nd one, the canonical structures will be the same (look at them from different angles, basically the same) and in a sense we can say "no resonance has occurred". For the 1st one, they will be different and hence will contribute to the resonance hybrid.
Not sure
Dear Satyanarayana
Can't positive charge in 1 can migrate to the tertiary one?
@pritish : why a hunch ?? identical canonical structures have a much higher resonance energy .. derfore the second carbocation will be much more stable than the first .
I tried to find the resonance structures of 1 but I couldn't get.Please any one of you,could you show me?So that I can know whether the structures are same or different.
@Asish : If that's the case, I have long forgotten what resonance is :P
hmm .. den it seems ive fr8en ochem ......... a try in abt 6 months :P
second one seems to be quite obvious becoz of resonance stabilization ....ain't it ?
of course its 1 since the oh group of the first benzene is higher in .........
the 2nd will be more easily dehydrated because the carbocation which will be formed by the departure of H2O will be resonance stabilised.
but in the 1st case the carbocaotion will not be resonance stabilised as the vacant p-orbital and the pi-electron containing orbital are perpendicular to each other....and for resosnance u need parallel or almost parallel p-orbitals....
There is resonance possible in 1st case, after hydride shift.
but still second one is more stable than the first one cuz there the carbo-cation so formed is 3° while in 1st case it gonna be 2° carbo-cation....
sry....resonance will take place in the 1st one but in the second case hyperconjugation is more....btw,i dnt hink hydride shift takes place in the rds....