second and third both should be ethene na!! least stable alkene is generally formed
21 Answers
Nah wait...that ain't Cope rearrangement. Those are for 1,5-dienes.
It's a sigmatropic rearrangement even then, a [1,5]-rearrangement to be precise. It follows a six member transition state. Check this for more info : http://www.chemtube3d.com/Sigmatropic4.html
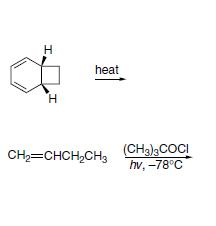
The first one is a Cope rearrangement and the second one seems like a radical reaction...
Mechs in a while...I'm not on the computer but on phone WiFi.
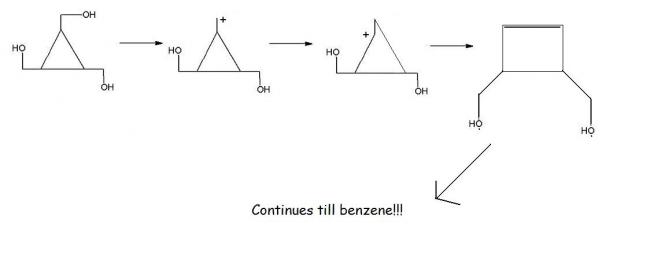
@Vinay : Ring expansion occurs if -
1. Energy is provided
2. The end product is either thermodynamically or kinetically favourable. In this case it is the thermodynamic factor, especially the stability which arises due to aromaticity.
After a nice long nap, I see that qwerty has beaten me to it...bleh :P
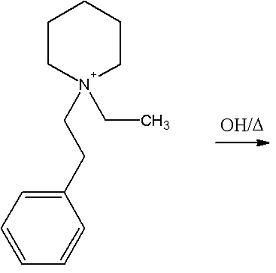
Quaternary ammonium hydroxide in which one of the substituent group attached to nitrogen atom has at least one " β " hydrogen when heated , undergoes decomposition to generate an alkene . This is known as " Hoffmann " elimination.
but how do i predict whether the compound will show hoffman elimination or zaitsev elimination?
3 > Same reaction - Final Product -
Only the cyclohexane ring will break and a double bond would come at the carbon atom immediately next to " N + " .
2 > This is Hofmann Elimination obviously . Elimination will take place at " β " carbon . Final product -
" C H3 - C H = C H2 + N ( C H3 ) 3 " .
All three questions look kinda familiar...lol. I'll try these tomorrow after getting back from college.
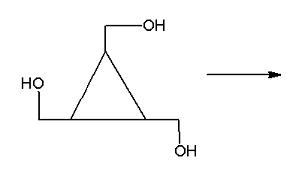
 ee if I am right:
ee if I am right: