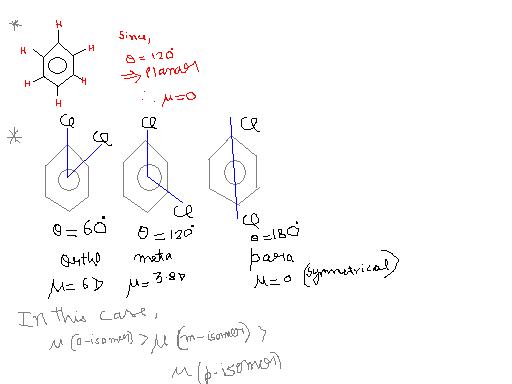
Dipole moment in aromatic system
can any 1 could explain?
THANKS in advance
-
UP 0 DOWN 0 0 5

5 Answers
Tush Watts
·2009-08-28 20:44:34
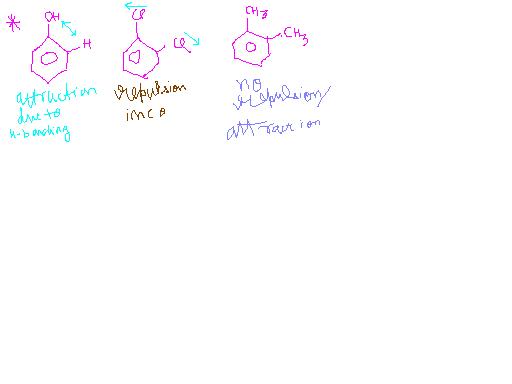
This is due to dipole -dipole repulsion in ortho isomer that inc bond angle geater than 60° and thus dipole moment dec.
theta dec, dipole moment inc
Dipole moment dec, no change in actual value.
Tush Watts
·2009-08-28 20:47:53
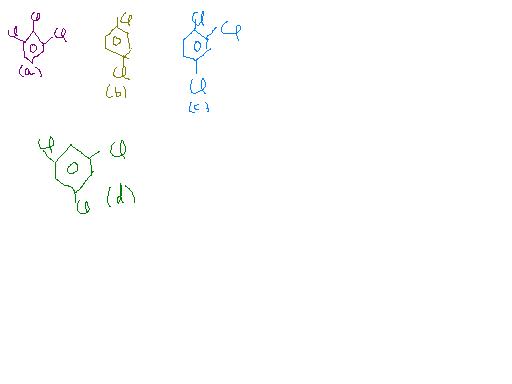
(b) and (d) are symmetrical and thus resultant dipole moment is zero.
In (a) bond moments are towards the same direction but in (c) there is a net dipole at the c2 position. Thus the dipole moment of (a) is max.
(b) = (d) < (c) < (a)
Asish Mahapatra
·2009-08-31 03:31:13
tushar:
with reference to #2, the dichloro substituent has higher dipole moment not the dimethyl substituent one