b
3 Answers
msp
·2009-06-29 05:49:28
Under acidic conditions, the alkene double bond is cloven to give the appropriate carboxylic acid:
CH3(CH2)17CH=CH2 + [O] → CH3(CH2)17COOH
Potassium permanganate oxidizes aldehydes to carboxylic acids, such as the conversion of n-heptanal to heptanoic acid:
C6H13CHO + [O] → C6H13COOH
Even an alkyl group (with a benzylic hydrogen) on an aromatic ring is oxidized, e.g. toluene to benzoic acid.
Glycols are highly reactive toward KMnO4.
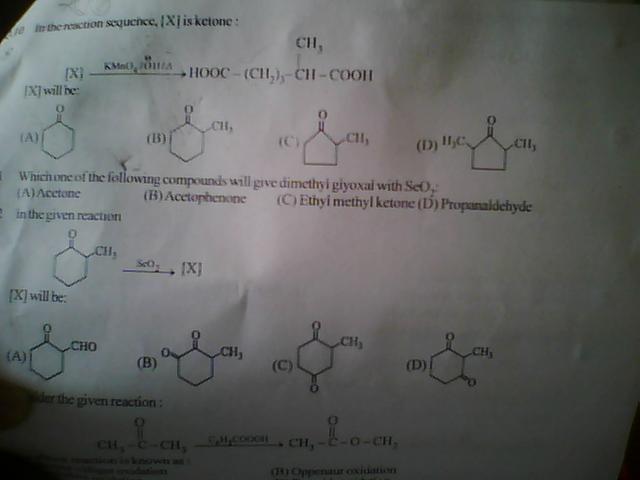 ...plzzzsolve q 10...top most
...plzzzsolve q 10...top most