ans 1 see the last line of given paragraph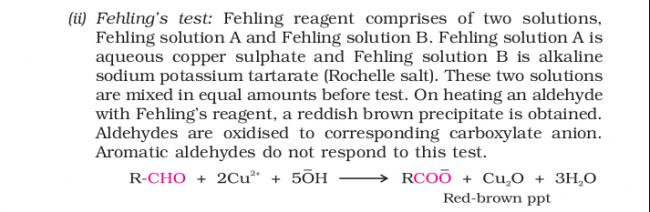
ans 2 red ppt.
1. Does Fehling’s solution oxidize Benzaldehyde? Or it oxidizes aliphatic aldehydes only?
2. .Aldehyde when reacts with fehling Solution gives
a) white ppt b) red ppt c) green ppt d) blue ppt
3. Which of the following gives iodoform test?
a) CH3COCH2COCH3
b) CH3COCOCH3
c) CH3COOH
d) CH3COCl
-
UP 0 DOWN 0 0 12

12 Answers
Shouldnt the ans of Q:3 be only a,b,.........why C......Does acetic acid give iodoform test?????
Ans 3) ONLY a and b. Acetic acid and its derivatives do NOT give the iodoform test.
In 1), Fehling's solution oxidises benzaldehyde so slowly we don't count it as a regular reaction. Infact we can test for aromatic aldehydes this way. We first react the aldehyde with Schiff's reagent, and get positive feedback. When we try Fehling's reagent, nothing happens, so we know it is an aromatic aldehyde.
oh yes,for 3 it will be only a and b.
the CH3CO group has to be present for iodoform reaction.
i'd better stop speaking and start strengthening organic chem again[7]
@pritish, from what i know, fehling's solution does not oxidize benzaldehyde, but DOES oxidize other aromatic aldehydes. So, the method u hav mentioned for detecting aromatic aldehydes can only be used for benzaldehyde.....if i'm not mistaken.
Well we have only benzaldehyde in our lab, so it may be like that..I was talking about a specific case then! lol
shreyan: Ive seen many cases where fehling's solution does NOT oxidise any aromatic aldehyde (exceptions when strong m-directing grps are attached at o,p positions wrt the carbonyl posn)
