But answer given at the back is (c)
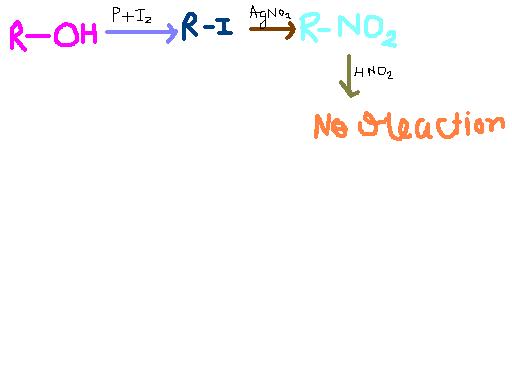
This reaction sequence can start with a :-
(a) 1° alcohol (b) 2 ° alcohol (c) 3° alcohol (d) Phenol
-
UP 0 DOWN 0 0 5

5 Answers
Most preferred starter would be a 1 degree alcohol....red phosphorus given here (it is usually red phosphorus) reacts with iodine to form PI3 insitu(in the reaction mixture itself), which like PCl3 gives SN2 reaction in presence of pyridine, or internal SN in absence of it. Usually pyridine is involved, isliye primary alcohol is the best bet. The next reaction is with silver nitrite, silver will abstract iodine to form a precipitate in all conditions, leaving a carbocation whether it is stable or not. This is because precipitate formation causes equilibrium of reaction to go very far forward, and silver has an unusually high affinity for halogens. So a 1 degree carbocation is not a problem here.
yes, the answer is (c)
....for1 °alcohol we get red colour due to the anion of nitrolic acid ! the acid itself is blue !
....for 2° alcohol the blue pseudonitrol is formed which does not react with NaOH
Oh..lol I missed out the last reaction's significance.
organic iska doubt 1 bhi check kar le yaar. I may have gone wrong somewhere, I'm half asleep as it is.