Qwerty yer right..for terminal alkynes, it forms a ketone.
Ques 1) 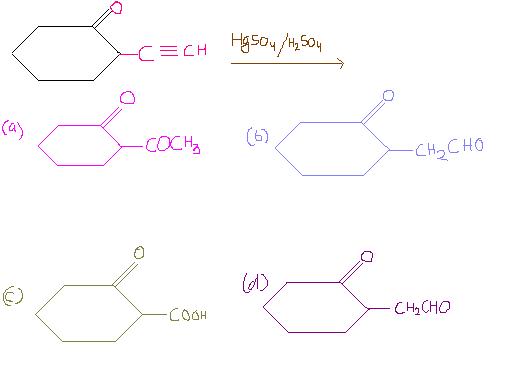
Ques 2) Following synthesis is effective when
CH3CO C l + R2 Cd → RCOCH3 + R Cd C l
R is of the type :-
(a) (CH3)2 CH - (b) (CH3)3C CH2- (c) (C6H5)2 CH- (d) All of these
-
UP 0 DOWN 0 0 13

13 Answers
Q1 - a ???
i donno da mechanism , but a shortcut .
It is addition of H2O by MRKR.
Then most probably it seems to be keto-enol
2) ka toh d) theek hai na, Dialkyl Cadmium....all are predominantly aliphatic...
@ Avik, dunno, assignment mein options (b) and (d) same diye huye the.
btw answer for ques 1 is (d) for sure (pakka 100%) [6]
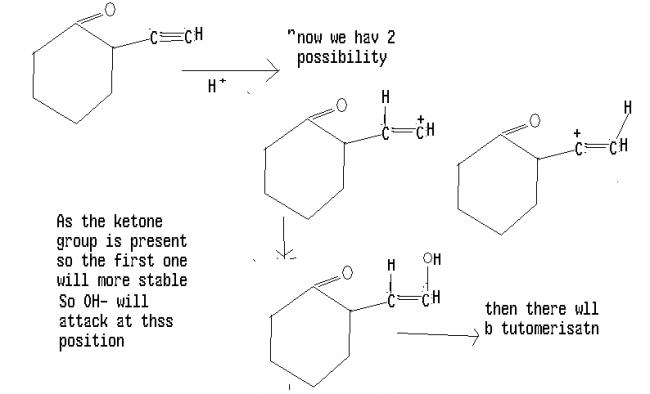
Ques 1 is not a lollipop, it's a special/ exceptional case. PLEASE koi to help karo. [2]
1) i too agree with swaraj. i find no other reasoning possible.
just that the keto group destabilises the intermediate carbocation.
2) this reaction is only possible , when R is 10 alkyl or aryl. ( for both cadmium and lithium salts )
well swaraj has made a mistake...he has formed a carbocation
whereas the reaction doesnt proceed by carbocation
thats why this reaction is rearrangemet free
i feel ans should be a
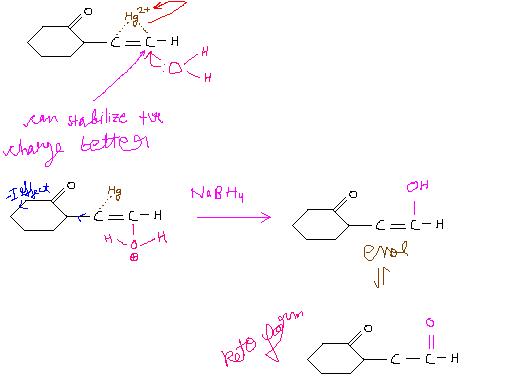
a detailed mech is ::
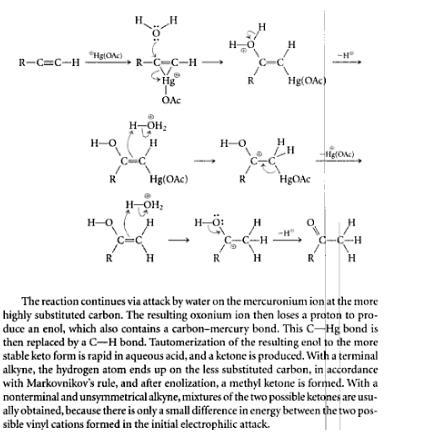
see as in the mechanism mentioned above a ring is formed....bt the ring gets broken frm dat side on which the positive charge is more stable.....
and so according to me the positive charge will be more stable on the outer carbon atom so the product will be b and d(as both r same)