Yeah even I think it should be E2 / SN2 .
But ans given is E1 / SN1
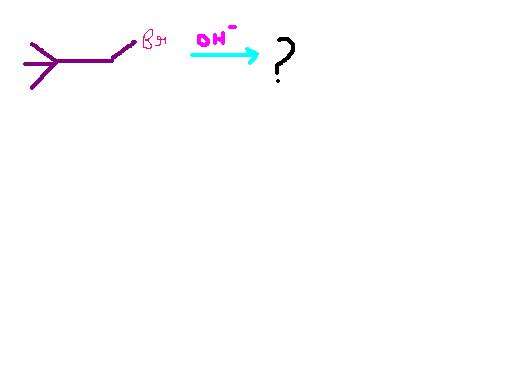
Just want to confirm it is E1 / SN1 or E2 / SN2 ?????
But it should be SN2 / E2 only. Here E2 will be dominant coz the substrate is very hindered and substitution is not feasible. The base is strong, therefore E2 > E1. SN1 is not possible at all.
First of all the alpha carbon is primary. Hence we check bimolecular mechanisms first, SN2/E2. Supporting this is the strong and unhindered base.
But the beta carbon is bulky, being tertiary, SN2 is ruled out. E2 should be the dominant mode of attack. Should. What we then see is that we have no beta-hydrogens. We can't facilitate an E2 reaction without those.
Slip down to E1/SN1. Once the bromine atom leaves, the carbocation rearranges to form a tertiary one, and then the base being unhindered attacks. The final preferred mode will be SN1.
oh yeah, Correct.. didn't thought of trying this by writing it down. E2 is ruled out correct... But pritish, how will you compete E1 / SN1 in this?? By temperature? E1 holds the possibility too..
Arey yaar I forgot to add one line : E1 is also possible if hydroxide ion abstracts a hydrogen..lol
now.. no.. nahi yaar.. E1 will not be possible.. considering OH- as strong base will not yield us E1 product, so in this question, we will consider nucleophilic character of OH- and that's the reason, it is used in SN1 innit.. including the fact that we have eliminated possibility of E2 / SN 2. SN1 holds more chances than E1 because of OH- weaker nucleophiclic char as compared to basic character .
What you say?? haa
Substitution = Stable nucleophile/Delocalised charge
Elimination = Localised charge/Strong base
Hydroxide ion is a strong base...
see gyz , u r already confirmed this reaction 2 undergo SN1 / E1 pathway. then hw com u r discussing abt the nucleophile's strength ?
u must be knowing that the Rate determining step involved here is dissossiation of alkyl halide frm substrate. thus , this pathway is independent of reactivity, strength and concentration of the nucleophile.