1
1********DONT SEE THIS THIS IS WRONG****************
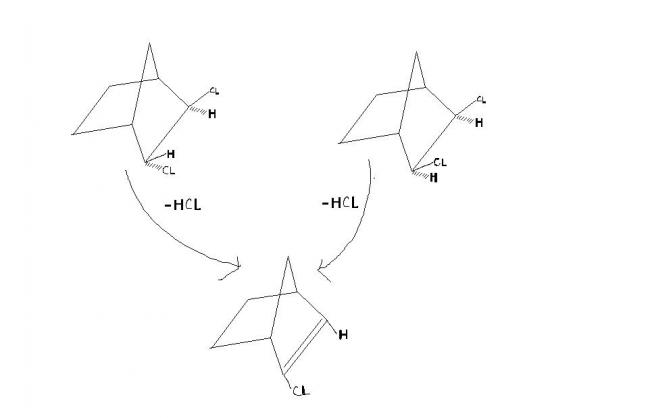
if the ans has to b given then i m with 1st reaction as both H,Cl r antiperiplanar
bt in reality i thnk that reaction will not take place in both cases (if structures r correct)
 11
11The bonds aren't so long as has been given in the pic. So the two chlorines being on the same side (plane of your monitor) create steric hindrance and so make the elimination easier [4]
P.S:
Just ignore the answer if it seems too far-fetched or idiotic [1]
 1
1yup thats wat i was 1st thinking b4 seeing the product but i cudnt get the structure of the product from reaction 2
 11
11Why not? The carbon becomes sp2 hybridized and so the hydrogen has to come to the plane of the monitor. Done! [1]
 1
1ohhk i got the 1st rection wrong H,Cl r antiperiplanar in 2nd reacton so ans shud b 2nd