16 Answers
SORRY...I DID NOT SEE THE ANSWER FROM ASISH..I WAS UPLOADING THE IMAGE !!! [1]
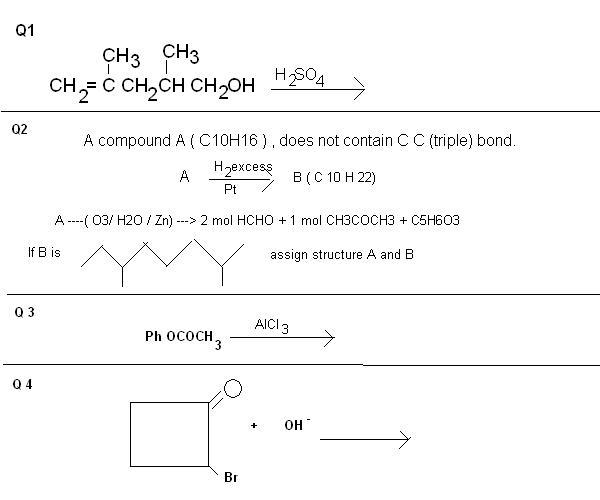
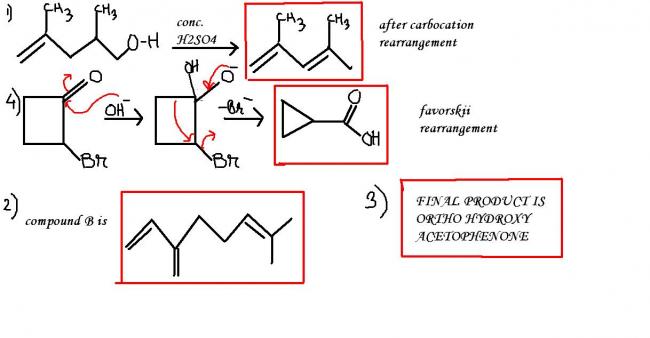
Yeah good.. Q2 is right.. but let anybody else try Q1 and Q4 then I'll tell if your answer for Q1 is right or wrong. Please try Q4.. I'm very confused in that question.
Alas it is not that straight forward.. if that had been then I wouldn't have given that question. Though I know Q3 is stupidity.. I didn't read that chapter yet and tried to solve that question. :D .
Given a singe hint and you will be able to do Q1..
Do try Q4
ankur bhai mujhe bhi 4th mein favorskii rearrangement hi lag raha hai...alpha bromo ketone, immersed in basic medium. and it is a ring as well. what is so confusing?
Debo is right about Q1 as well, ek rearrangement ke baad HSO4- anion will take up the proton and a diene will result.
Good pritish.. vaise.. I didn't think of Favorskii in that question at all (silly me) but answer is not of favorskii.. favorskii rearrangement should only be the case when the contracted ring can bear the strain upon itself.. here already cyclobutyl ring is too strained and after it will get converted to cyclopropyl ring.. certainly it's gonna pissed off... thanks for making me remember about Favorskii.. Answer of this question is based on Sn / E.
For Q1: It's not like this... I lingered with this answer for a bit but when I checked my answer I was wrong. There is something else going in that reaction. Think of it... there is an alcoholic group and olefinic carbon..
Thanks for participating and answering everyone ! :)
are u hinting at the possibility of a lactone ring in question (1) ?
hm.. you got it!
vaise.. can you clarify Q3? I mean, no problem with Favorskii rearrangement but in this case neither answer is matching nor I think that contraction to cyclopropyl group will be feasible.