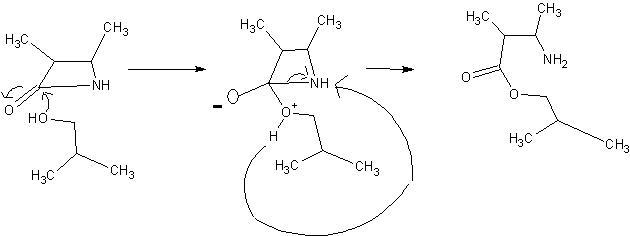
isn't a H there on N ?
assuming it's there . this should be it.
well , first we get an substituted amide , which on acid hydrolysis gives. these products. I think.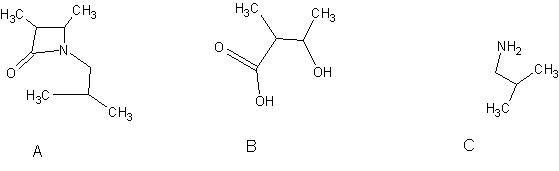
isn't a H there on N ?
assuming it's there . this should be it.
well , first we get an substituted amide , which on acid hydrolysis gives. these products. I think.
But options are not matching Ok i am posting the answer then also if u will explain it will be fine.
there is an attack on the electrophilic carbon . by the alcohol . I'll post the mechanism in a mo.
this should clear it. the hydrolysis part is easy , you should be able to do ti.