The structure is correct(as given) and answer for the 2nd ques is (c) only
1) Which of the following resonating structures of 1- Methoxy -1,3 -butadiene is least stable
(a) H2C - - CH=CH - CH =O + - CH3
(b) H2C = CH2 - - CH2 - CH = O + CH3
(c) H2C - - + CH - CH = CH - O - CH3
(d) H2C = CH - - CH - + CH - O - CH3
2) Which of the following is the most stable intermediate and why ?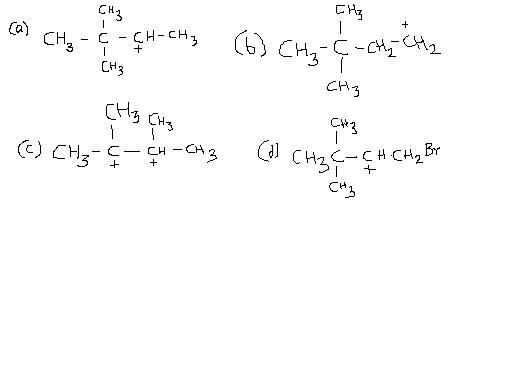
-
UP 0 DOWN 0 0 10

10 Answers
ans for (1) may be (a) because (i) positive charge is on oxygen atom (ii) there is less charge separation!
ans for (2) if the answer is (c) then the argument that comes to me is that the intermediate is a sandwich type that does not make it a flat structure(as is the case in bezidine rearrangement)....3° carbocation is a relatively minor fact here because for a flat structure the adjacent positive charge repulsion would override the 3° significance in leaps and bounds !
Yeah, answer for II should be c only.. because hyperconjugation is II most important criteria for seeing stability (after resonance).. and also, it is getting stable by inductive effect.