ANS : All four of them are Correct..... (gud one srinath !!)
& now comes the eXplanation part.....[1]
(B) & (D) am confident with; but how about (A) & (C).....??
@Ronald....didn't get ur point exactly buddy....
Multiple answers correct.
Select out the correct Statement(s) -
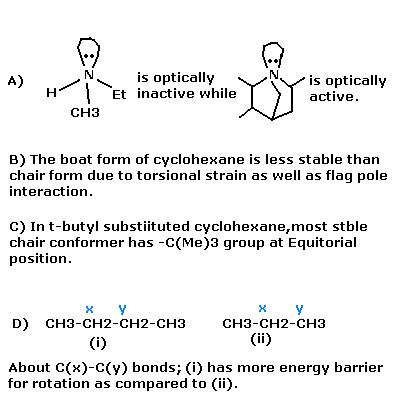
ANS : All four of them are Correct..... (gud one srinath !!)
& now comes the eXplanation part.....[1]
(B) & (D) am confident with; but how about (A) & (C).....??
@Ronald....didn't get ur point exactly buddy....
equatorial position has max available space.. so bulky groups prefer the equatorial to axial position .. hence (c) is correct
@D' (big name u got he!he!) ...But they can't exist individually at a time naa......so why this talk of individuality ?!!
Yup got it now ronald, thnx [1].....but confusion persists with option C)....
Yeah..they are reversible, but individually they are optically active..
Or I am toking crap?
ITS A REVERSIBLE SIGN IN MIDDLE..SORRY FOR THE WRONG ARROW..
avik u got it ?? i read about this in fiitjee material.
A) cannot be correct.
For the 1st compound,
Both d and l are formed. But they are interconvertable. Interconvertable at such a fast rate (can't recollect the time period) that they cannot be resolved.
A is correct for sure..
in 1st compound the lone pair oscillates from one end to the other,,
the compound and its mirror image cannot be isolated,as inversion takes place rapidly.
technically speaking this is Umbrella effect..the 2nd compound because of its rigid structure does not show such effect.
a,b,d are correct.a is due to pyramidal inversion.i dont know about c
hey guys give the reasons for your answers
anyways the answers are A,B,C,D I think
do correct me if I'm wrong
(A) looks true if the hydrogen performs the same function as an alkyl group !