Ans 5) If I am not wrong, abstraction of H atoms depends on the type of reaction like SN1 and SN2 na? you havn't mention about this.

For 1st and 2nd image
Question: what type of geometrical isomerism across the given double bond of the cyclic compound
For 2nd and 3rd
Find R and S using golden rule across each chiral carbon atom
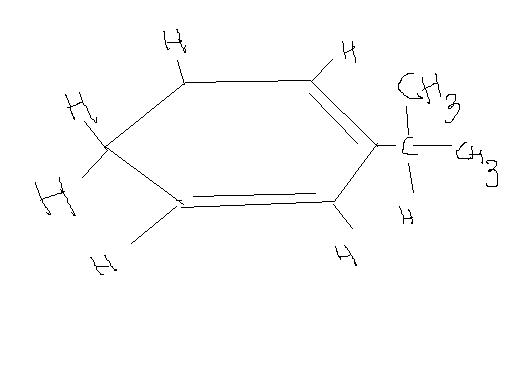
For 5th
Which hydrogen is abstracted more easily??
-
UP 0 DOWN 0 0 12

12 Answers
Can you be a little more precise in your questions?? Like which question wants what?
There's no double bond in 2nd image, for example.
In the 5th image, two hydrogens can be removed by dehydrogenation to form a double bond which results in benzene.
Nucleophilic substitution se thodi karenge...where's the leaving group?
And I was wrong about the tertiary carbon. Edited that part.
here abstraction is related to substituiton reaction
but not nucleophilic
in last one
ans is given
as
the
H atom attached to tertiary carbon
reason: allylic carbocation ( 3°>2°>1°)
even though am confused
Alle Kalyan Babu, [3][3]
1)reason yeh hai ki (c) and (a) has the same -CH2 group on the same
side of the double bond so they are cis isomers where as (b) has the -CH2 group on
the different side so its a trans isomer. (see its forming Z like structure)
In the 2nd one see, you have made the two groups with two different colors which means
that two different groups are attached to the same side so its a trans isomer
@ Pritish, I made a mistake[1][3]. I keep on doing stupid mistakes, thanks for telling[1]
@ kalyan , in the fifth image, carbocation wont be formed, instead carbanion will be formed
when H is abstracted, it leaves as H+, the electrons of C-H bond will move towards the carbon , making the carbon negatively charged
but dude carbocation isnt getting formed, so why shud we talk about stability of Carbocations at all? see post 9
No carbocation can form...you don't abstract hydride ions, you abstract protons..
I had thought of the 3 degree carbon as an anion which resonates between those two double bonds, but it resonates only with one. Rather two hydrogens can leave as H2 with some energy/heat given to the substrate and benzene can form...just a guess. But it would make sense.
