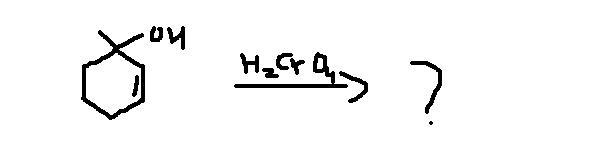106
106Oxidation of a tertiary alcohol requires vigorous conditions such as high temperature. (usually high temperatures are used even if alcohols are not tertiary as well)
Now at high temperature, the kinetic factor becomes less dominant while thermodynamic effect assumes importance.
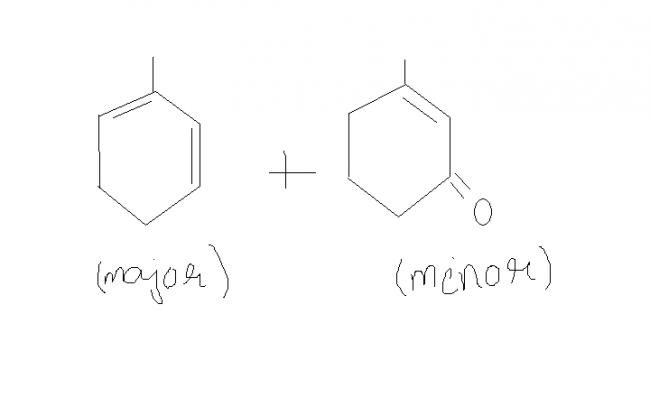
So, tushar's product is the major product as it is thermodynamically more stable (as the intermediate is more thermodynamically stable)
 1
1hehe .. nice
but don't waste your time on curtin -hammet not at all useful for JEE .
try not to waste time now.
do more important stuff first .
regards
 24
24and more importantly..i ma beginning to understand Curtin–Hammett principle now
 1
1any of you guys who maybe interested may go through this
http://www.chemicalforums.com/index.php?topic=38786.0
It doesn't matter much if you don't understand everything ,
yet if possible try to and wrt the question going by the
discussion in the other link , I guess one can safely leave it out for JEE .
 1
1hey guys
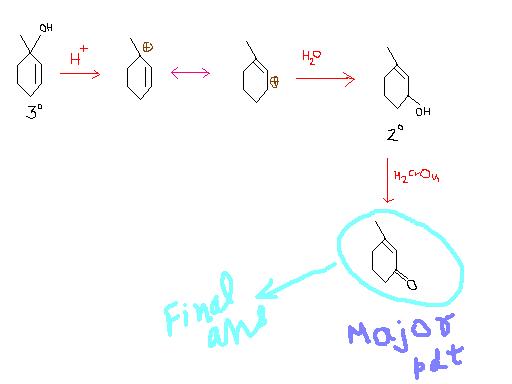
it I guess won't go through the mechanism shown by tushar .
major product shown is right but the mechanism is different .
I myself don't know the mechanism will let you guys know if I get to know.
 39
39Asish is right! Higher the temperature, the thermodynamic pathway becomes more favoured.
One little known fact about carbocation rearrangements : They don't occur according to energy stabilities, a 3 degree cation can rearrange to a 2 degree cation AS WELL. We view the energy stability factor so as to obtain the product which will be in maximum yield. It is incorrect to say that a 3 degree cation cannot rearrange to a 2 degree one. Rearrangements keep occurring, it is the most stable intermediate which we say yields the major product, that's a different story.(Source - Solomons)
We normally take into account thermodynamic factors there. Kinetically, the product in which resonance(pi bond shifting) doesn't occur would be major. But at high temperature Tushar's product is correct.
And happy new year everyone. Been a while since I posted here :)
 106
106eure.. what abt the reaction of HCl (1 eq) with 1,3 butadiene?? at high temperature? Why does a 2° allylic carbocation rearrange to a 1°allylic carbocation?? Its the same reason (the steps are reversible)
 24
24@tush ,,how can ur mech be OK ???
3° carbocation will never rearrange to become 2°
 11
11@ aieee and eure
May be my reasoning is incorrect. But ans given is not wrong 4 sure. It's correct .
But 3° alcohols are however difficult to oxidize. But on oxn under drastic condition first gives ketones with lesser no. of carbon atoms.
 24
24i too agree with aieeee.....maybe ans is wrong then
thx all [1]
 1
1tushar , the mechanism u hv done is right. but why this is the major product.
ok. lets assume the ans. u hv given is the major product. thus , the transition state involved in your product is surely more stable than the other.
thus , the two transition states( carbocations) involved are :
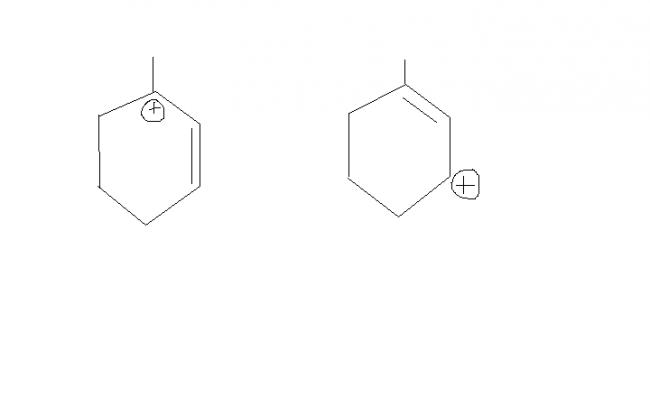
but , u cn see frm the fig. , 1st carbocation is more stable. reasons :
i) tertiary carbocation.
ii) allylic carbocation
iii) more hyperconjugative H's than the other structure
 24
24ya...same thoughts here....
and strange thing is that ...major product acc. to u is not even in the options..[2]
 1
1strange. its a conjugated product. why not major, can't think of any reasons.
 24
24ur minor product is given to be major