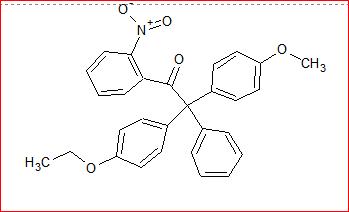
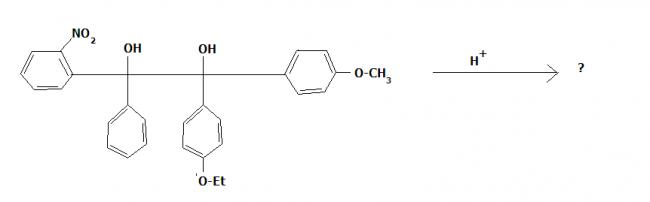
Pinacole Pinacolone rearrangement is whats comin into my mind!
3 Answers
Vivek @ Born this Way
·2011-11-03 23:08:36
Yes!, It's that only.
Protonate the right -OH group and let it depart in form of H3O+. Now to stabilize the carbocation generated the lower left Phenyl group (Unsubstituted) will migrate to that (Why?) and generate a positive charge at carbon. This will now be satisfied by the lone pair from oxygen generating a Ketone group.
It should look like :