there's a correction in the above question. I've written CH2Cl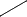 CHO. But it will be CHCl2
CHO. But it will be CHCl2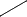 CHO.
CHO.
When HOCl is added to CH CH , CH2Cl
CH , CH2Cl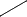 CHO is formed. But how is HOCl added to CH3C
CHO is formed. But how is HOCl added to CH3C CH ?
CH ?
I'm not sure about propyne as it is unsymmetric and whether Markonikoff's rule is used or not.
How is this reaction affected by the +I effect or -I effect?
-
UP 0 DOWN 0 0 5

5 Answers
prongs05
·2009-11-06 02:09:00
iitimcomin
·2009-11-06 07:56:16
basically make sure the carbocation intermidiate is most stable .... it will take care of every thing ......
prongs05
·2009-11-06 08:25:29
soo .. Cl should be added to the middle carbon and OH to the terminal carbon. Am i getting it right? and are u sure??