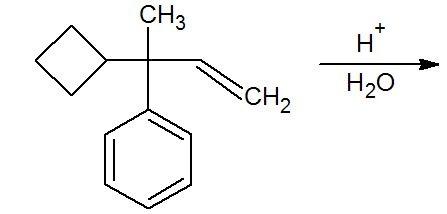
i clearly remember this monster of a qstn....
10 Answers
I have a doubt regarding the answer.. what answer are you getting?
Well the answer given is 2,6-dimethyl-1-phenylcyclohexan-1-ol.
Here's the process I got:
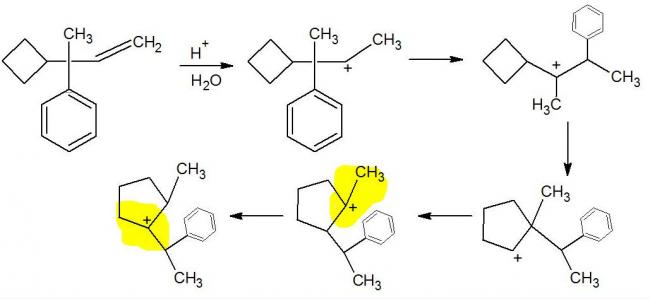
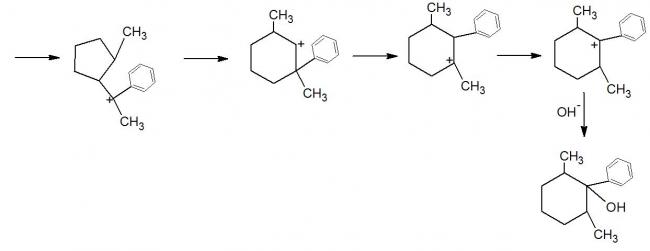
Now notice the highlighted steps.. don't you think this rearrangement should not occur because there is no increase in intermediate stability (from a 3° carbon to another 3° carbon)?
the left most highlighted step is ok....but the highlighted one in the middle dsn`t make sense....both leads to a 3-degree carbon,so the pathway depends upon the intermediate during the shift....
i feel even i was wrong....in the 4th last step,hydride shift from the carbon situated to the left of the carbocation,is most probable....H- shifts pretty fast....
actually,i dont knw how to draw,so i`m writing everythng....bit confused as well
I somehow get a feeling.. Rearrangement should stop at the highlighted position.. Waiting for some expert to validate!
i think hydride shift occurs to get the most stable carbocation....so the steps should follow....
Thats true.. But remember sir told us, we can't force a hydride shift without an intermediate increase in stability even if the step gives us the possibility of increasing the stability later..
Okay, it's been a long time since I've come here, so don't take to heart anything I say.
As much as I remember about carbocation shifts, they occur irrespective of the degree of the resulting intermediate. So a 3° to 2° hydride shift is possible, though it is the 3° intermediate which will actually last longer enough (due to its stability) to garner more product molecules (and hence the major product).
What has happened here is completely legit. It is one product out of many. It could've gone Ashish's way and stopped right there as well, but that intermediate is not stable enough compared to what we get at the end. What we want is the most stable intermediate as we know that it will constitute the major product. We know that a six-member ring provides exceptional thermodynamic stability. If a possibility of ring opening is available, take it and go along with the flow, especially if we will end up with a 6-member ring.
</end rant>
Thats all due to the angular strain in the four membered ring leading to phenyl shift .