remember C-C bond is stronger than C-H bond due to symmetrical overlap of orbitals ! hence breaking it is tougher than the other !
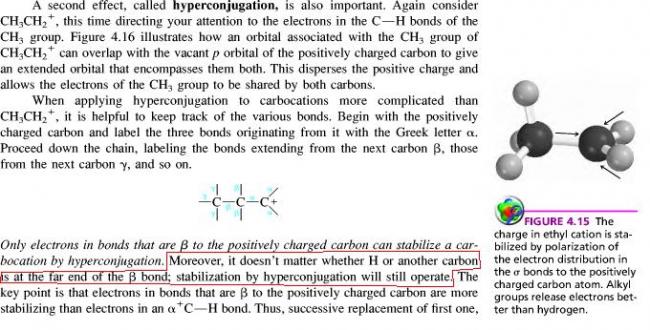
From Carey.
explain that circled line.
C-C bonds also involved in hyperconjugation??
So, is C-C hyperconjugation more stabilising or C-H ???
-
UP 0 DOWN 0 2 4

4 Answers
Debotosh..
·2009-11-30 23:00:29
voldy
·2009-12-02 23:17:45
I'd like to ask one question
What according to you guys is hyper-conjugation exactly ?
Remember one thing the bond is never broken completely even in the case of H .
The proper explanation comes in MO theory , if anyone is interested do a google
and btw why is ethane in the staggered configuration ??? and not eclipsed . now don't say steric strain ....