am sry .... definitely out of touch ... didn't remember to consider the factors ... directly answer likh diya ... dumbo me [3][3]
1. Reaction and mechanism of :
CH3-CH=CH-CH2-OH ----------------> (A)
SOCl2
Find (A) and it's structure?
-
UP 0 DOWN 0 0 16

16 Answers

so i thnk accrding to this (1) shuld be CH3-CH=CH-CH2-Cl
am a little out of touch so nt sure if this is 100% correct or not .. verify with the given answer pls..
for 1st is it CH3 - CH(Cl)-CH=CH2?
2nd question seems wrong N cant have 5 bonds
First one involves carbocation formation so there will be resonance (SOCl2 without pyridine). I guess qwerty's answer is correct.
2nd question seems wrong to me too...unless that's a diazonium ion.
how to find spring constant wheny a spring is into 2 pieces.i.ek of the cut out springs.
given only spring constant of the parent spring.
@Richard: I would have loved if u had started a new thread but anyway! :)
See first of all I am discussing this for an ideal spring
An ideal spring is massless and thus, whatsoever the acceleration be, the tension at each and every point of the spring is the same and that is equal to the force with which the string is stretched from the both ends! :)
Next see..
If we mark colourful points at various points of the spring and fix one end and pull the other end, we will find that the farthest end moves farthest and closest end from fixed end moves smallest (easy to logic out why.. divide the spring into n parts of equal length L and due to expansion each of these part expands equally since tension on either end of each is the same and new length becomes L+a..now try to convince yourself)
Also we can state, the distance moved by the colourful points is proportional to the distance of the point from the fixed end...
Initially for parent spring of length L, and spring constant k,
F=kx.
Now consider a spring part of length a..
By previous logic, the force pulling this segment from either end (tension) is also equal to the force F.
again by previous logic, the length l will expand into aLx...(expansion is proportional to x)
Now if we cut out this length l, and apply an equal force F to it and consider it's spring constant to be K' and consider it's expansion to be x'
we can write, F=K'.x'
but we know, x'= aLx
or, F=aK'Lx
but we knew, F=kx
So, by comparison,
k=aK'L
Or, K'=LaK
I hope that you got it! :D
Gosh! I still remember this! :D :D :D
@ALL,
Yup, the first one is correct, and please I need the mechanism for that. Also, What type of reaction is it?
Second One: It is correct. I have drawn it in CHEMSKETCH Software and I don't think it will allow me to draw wrong structures unless the software is faulty.
Vivek : Check my notes on Alkyl Halides. You'll get it in the preparation of alkyl halides from alcohols.
As for the 2nd reaction, I can't remember it no matter how hard I try...:(
ignoring the N with five bonds
ch3o- will attack where extent of ∂+ charge is more
O2 is more electro negative than N2
so polarity of C--O bond is bore.. so the neclueophile will attack on this carbon
don't laugh ;)
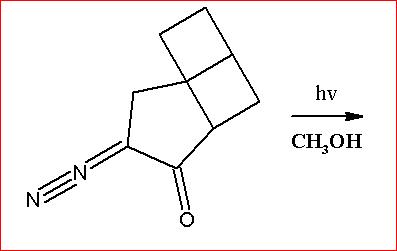
Indeed subho...there is a huge significance of light here...it causes some sort of decomposition which I cannot recall.
Anyways, I managed to recall ( or better say find)
" hν " causes thermalysis or Photolysis of Diazomethane group which liberates N2 and generates a carbene species.
Upto that part is okay, But does that carbene react onwards?