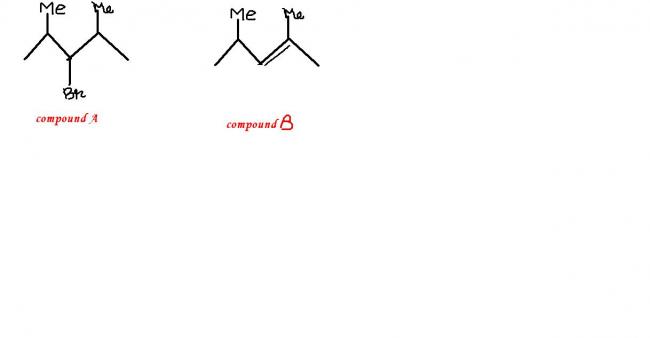
Yeah.. good. Answer is correct. But I'm wondering why it can't be: 2-bromo-2,4-dimethyl pentane.
Q: Compound A (C7H15Br) is not a primary alkyl bromide. It yields a single alkene B on being heated with NaOC2H5 / C2H5OH. Hydrogenation of B gave 2,4-dimethyl pentane. Identify A and B.
PS: Scroll down to meet few more questions.
-
UP 0 DOWN 0 0 11

11 Answers
Because it says only one alkene is obtained on heating. Had it been 2-bromo-2,4-dimethyl pentane, you would have 3 different possible alkenes on heating.
ohk. But one thing more.. This states that reaction is E2. Why the reaction can't be SN1? C2H5O- is strong base and solvent ; C2H5OH is polar protic. Can anybody enlighten this combination for me?
Q 2 --> 2,4-dichloro but-2-ene reacts to give NaOH to give A (major product). Identify A.
Q3: 3-bromo, 1-3methyl pentane reacts with C2H5ONa in presence of C2H5OH to give A(major product). What is A?
Q4: Which condition/reagents would you employ to obtain best yields in the following reaction:
2 bromo butane ------------------> butene
a) t-BuO- / BuOH, Heat
b) C2H5ONa/C2H5OH, Heat
Substrate is secondary, base is strong. Thus elimination is favoured here. Had it been a weak base, SN2 would be the followed path.
Q3 mein E2 hoga, the base is strong but the substrate is tertiary. Due to strength of base, unimolecular mechanism is cancelled out.
As for E2 : 3 > 2 > 1 (Hyperconjugation)
And for SN2 : 1 > 2 > 3 (Steric hindrance)
Thus SN is also cancelled out.
In Q4, I would use tert butoxide ion. It is sterically hindered base and will prefer elimination more than substitution, with sodium ethoxide there is a slight possibility of SN.
DIFFERENTIATING A BASE AND A NUCLEOPHILE
1) electron density is max on a base...thus for some given species,the species with greater electron den performs the role of a base while the other becomes a nucleophile
2)e/r ratio- size factor-...this is max for a base and min for a nucleophile
3)polarisabilty --high for nucleophile and low for base
...thereby we can say that a bigger atom with smaller e/r is more of nucleophilic character than basic character , while a smaller atom with high e/r is a better base than a nucleophile !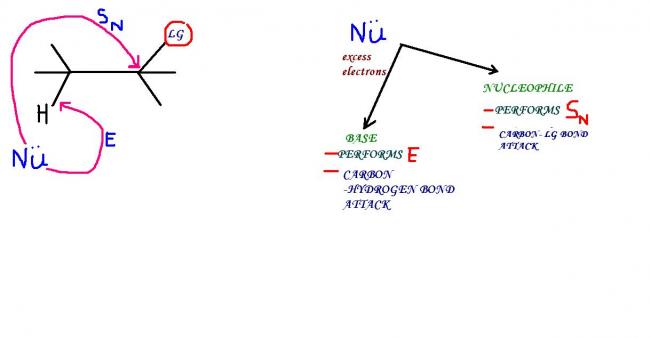
i think this is an explanation able enough to clear your doubts, mr ankur !
Thanks for answer but I reckon I already knew this stuff. Perhaps reading the question is required once more. Inspite of Nu vs Basicity; I was asking for the action of strong base in polar protic solvent. It's cleared to me.