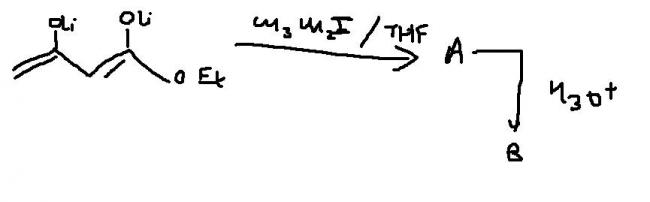just , note that how the delocalisation on the right is done. carbon containing O-Et group is attached to two oxygen rather thn C.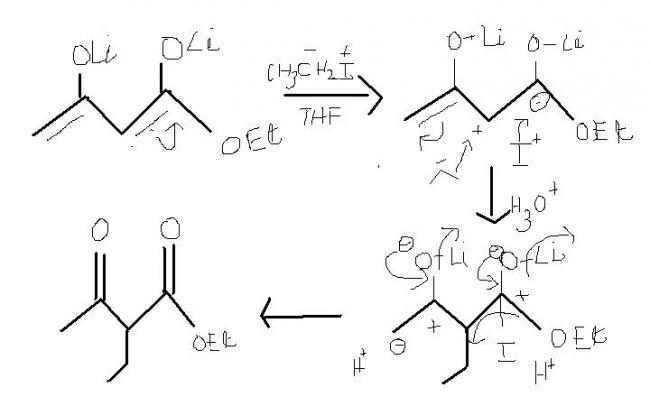
3 Answers
aieeee
·2009-10-08 19:50:35
aieeee
·2009-10-08 20:13:46
doubts u may hv :
i) by the delocalisation at the right , at the middle ,secondary carbocation is formed as a result of delocalisation . if it would hv been other way then , primary carbocation would hv been formed. ( cn also judge on the basis of stability of carbanion. )
ii) Addition of I in the form of I+ but elimination in the form of I- as it is a good leaving group.
iii) O-Li group or O-Et groups can't hv been directly substituted or eliminated.[9]
eureka123
·2009-10-08 20:17:43
really nice work....
and u answered all the dbts that came to my mind while reading the mech too[4][4]
btw is this some named reaction ????becoz it has a strange mech [12][12]