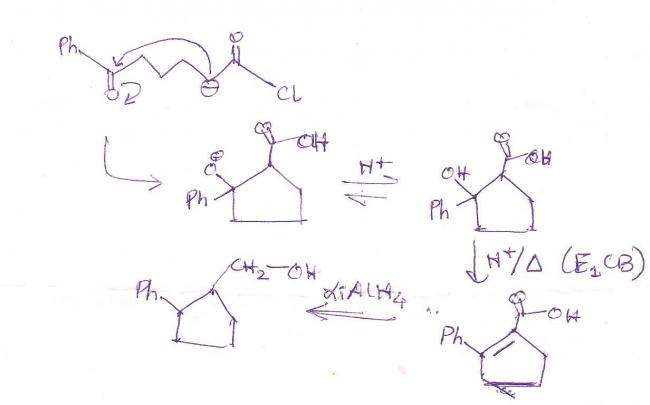
I have a bit of a doubt in this question...about whether it follows the regular aldol condensation mechanism. Hydrolysis/heat is the last step of the aldol condensation, after the ring has formed. But here, there will be no -OH for that...O- will reform its pi bond to make C=O by expelling -Cl as a leaving group. So what purpose does hydrolysis and heat have?
For your grouse with LiAlH4, it reduces double bonds only if -Ph is at a position beta to the double bond. By regular aldol, the double bond would be alpha to the phenyl group.
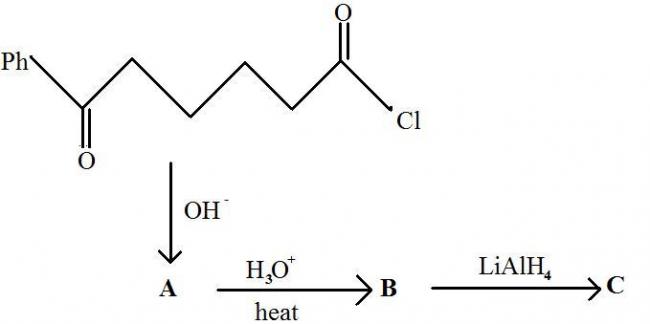
my answer: 2-phenylcyclopent-1-ylmethanol
given answer: 2-phenylcyclopent-3-ene-1-ylmethanol
am i correct??
if not explain by which mechanism LiAlH4 reduces! :)
thanks in advance! [1]
-
UP 0 DOWN 0 0 5

5 Answers
Pritish Chakraborty
·2011-01-21 10:08:14
AKHIL
·2011-01-21 21:15:22
u must be doin some mistake in conversion of B to C
otherwise it is aldol condensation only!!
Subhomoy Bakshi
·2011-01-22 05:13:33
i think it was in FIITJEE Archive...i do not remember..or might be arihant! :P