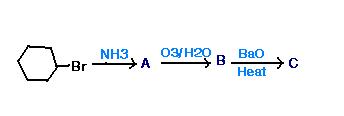
will a double bond be formed in A??
12 Answers
Ammonia is a decently strong base. It would abstract a proton primarily. Ozonolysis in the next step indicates that elimination should've occurred in A.
Yeah, but if there's a st. chain, NH3 replaces Br 2 give an amine lyke -
RCH2Br ----(NH3)---> RCH2NH2
Then why/how wud it deprotonate here ?
& wat does BaO do ?
it's simple. A is cyclohexene, then ozonolysis gives: succinic acid. Then BaO gives Pyrolysis reaction .. and hence cyclopentanone.
I'm 110% sure of my answer. :)
Yeah rite... Pyrolysis reaction !! (Is it a part which is taught under Carboxylic acids?)....
n how is this Cyclohexene being formed ?
Haan pyrolysis of esters and carboxylic acids is the topic.
Cyclohexene is formed by simple E2 elimination. The substrate is secondary, and elimination will be favoured for secondary substrates when a strong base attacks. If the base would have been weak/stabilised, substitution would've occurred.
NH3's nucleophile character is dominant in case you encounter with Primary halides and that's why favors substitution, while with cyclic / secendary and tert halides / groups, it's base character is dominant and favors elimination.
And.. about pyrolysis, I read it in Carbonyl.. and yeah it was in carboxylic acid too.
@ ankur...are u sure succinic acid will form after ozonolysis of cyclohexene? (succinic acid has 4 carbons)
oh yeah.. sorry! messed up while naming that.. arbitrarily typed that word.