asish ur post 19 clearly explains everything :)
19 Answers
my vote goes for water
but asish and his sir say otherwise
also wiki and see the pka values
morrison boyd AND solomons give CH3OH as more acidic
quoted from morrison:
Just how acidic are alcohols? With the exception of methanol, they are somewhat weaker acids than water
quote from solomons:
As we might expect, alcohols have acidities similar to that of water. Methanol is a slightly stronger acid than water. (pKa=15.7) but most alcohols are somewhat weaker acids.
explanation as provided by BT (i had asked them) i am not quoting this one just writing in brief
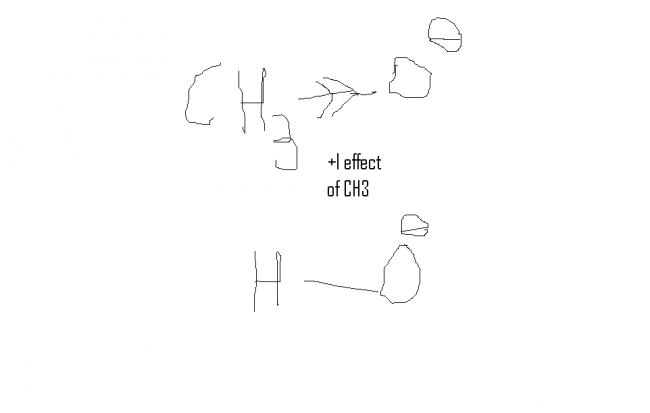
Alcohols are weaker than water because they are sterically hindered. Hence they cannot be easily ionised. However, methanol CH3OH having the least steric hindrance is approximately same as water. Its acidity might be explained due to the fact that as its steric hindrance is less, the ions that are formed become solvated resulting in more stability of the methoxide ion. But this is not true for higher alcohols as more +I effect tends to destabilise the alkoxide ion and also as steric factor increases for them
its water sure i was having that doubt which i got clarified from my sir
Rahul ur explanation is fine
but how abt the pKa values
any chem expert here ??
pKw water =14
pKa is for rxn
H2O → H+ + OH-
Pka =14 + log[H2O] = 14 + log[55.5555] =15.74
i think it is water
as when we goto conjugate base of H2O which is more stable than conj. base of CH3OH which is de stablised by +I effect of CH3
below fig. might helps
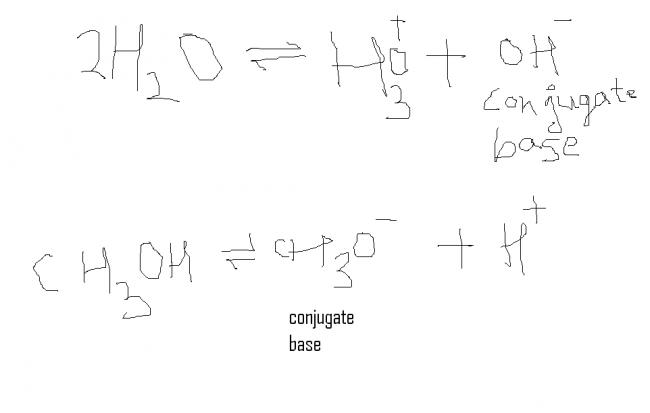
it depends on temperature
Ankit it is 14 at 25°C
its pKa value changes with temperature
MAYBE I THINK I POSTED A CLEAR REASON+NCERT QUERY IN CHATBOX ITSLEF
Celestin.... pka methanol=15.5
Celestin..: pka water =15.74
Celestin.._: im confused now :(
john cen.._: @kamalendu methanol is more acidic i have read it many places n reason is also easy
Celestin..: oki also read in NCERT all alcohols less acidicthan water
kamalend..: @ celeoh! yes so did i
john cen..: see acidic nature for alcohol depends on the alkyl group in alkoxide ion,if it is more bulky,there is less stabilization of alkoxide ion
Celestin.._: yes i agree but y is it gvn in ncert all alcohols less acidicthan water
john cen.._: NCERT says alcohols,are however weaker acids not ALL ACOHOLS...........
john cen.._: FROM STATEMENT IN NCERT IT IS CLEAR IT IS A GENERAL STATEMENT REGARDING ALCOHOLS
Celestin.._: ok i guess its not imp for jee ??
Celestin..: thats wat asish's sir told
john cen.._: but that we can conclude from simple rules of deciding acidic character na.................isn't it easy
wait for tomorrow i will scan u a page from my organic book where it clearly says that water is more acidc