@ ASISH ,I HAV DOUBT IN Q1. THT WHY D>C
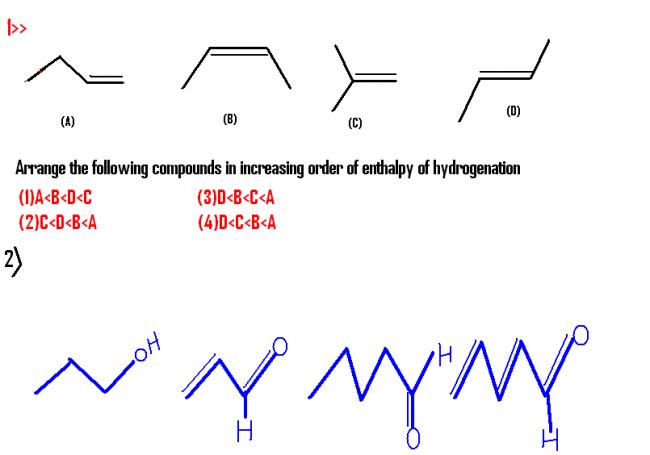
QUESTION 2 >WHICH OF THE COMPOUND HAVE HIGHEST DIPOLE MOMENT?
-
UP 0 DOWN 0 1 6

6 Answers
Ankur
·2010-02-05 21:40:44
for Q1: lower the enthalpy of hydrogenation, stabler the alkene.
Moreover, 'An alkene is stabilised by alkyl substituents on the double bond". This means C is the stablest among'em all. That's why consequently highest heat of hydrogenation.
swaraj jena
·2010-02-05 22:21:55
ur answers r correct asish
my doubt z due to which effect C z more stable thn D