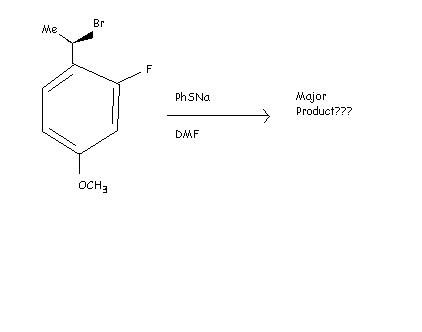
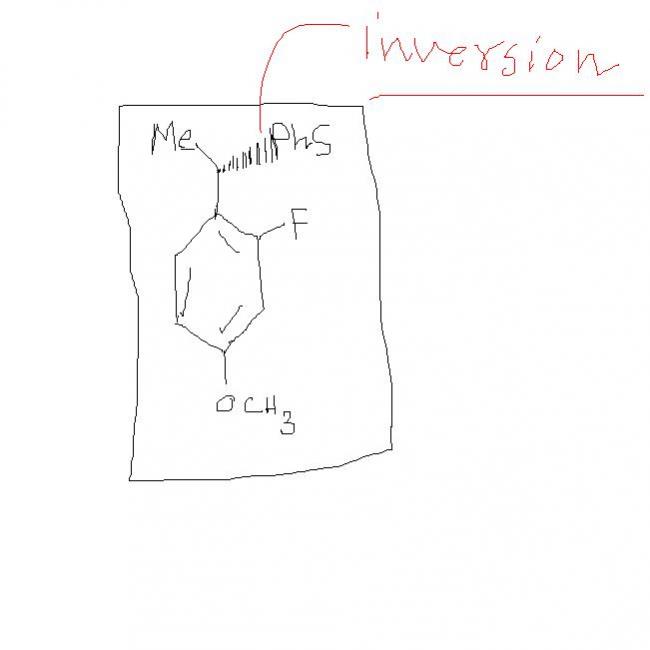
actually the bromine at the benzyllic position gets replaced by the nucleophile :PhS-.....the most important part of the reaction is that nucleophilic substitution of the halide atom can occur only at the alkyl region.....that is why we are replacing the bromine and not the fluorine atom that is linked to the benzene ring by strong partial double bond !!!!
19 Answers
IF GOOD BOOKS SAYS THEN IT MUST B RIGHT...THATS WHAT I BELIEVE...BTW WHICH BOOK??
sky....r u sure that inversion will take place even in the presence of a activating group in the ring
plz.....explain a bit....what should be the correct ans....i have no idea[2]
possible....since electron density increses at para so may be that helps in carbocation forma.....
but...book says different.... due to activating group there will be retention of configuration.....[12]
ABHI INVERSION IS INDEPENDENT OF WAT'S THER AT PARA POSN.....
I GUESS IT JUS DEPENDS ON DA NATURE OF REACTANT AND ITS TYPE
in JEE 2008 there was -NO2 instead of -OCH3
Will inversion take place ?? there is -OCH3 group now???[7]
this is jee-2008 question i think...
PhS- will attack Br from 'back-side' ..
DMF helps in SN2 reaction ... so final structure will be ...
no SNAr not possible which leaves only two possible competing reactions . which are they ?
DMF- DIMETHYL FURAN
PhSNa- phenyl and sodium attached to sulphur.
source for PhS--