Can some1 give a better explanation..
What is the easiest way to differentiate between Metamerism and Position isomerism??
Kindly explain..
-
UP 0 DOWN 0 0 6

6 Answers
In position isomerism a functional group changes position on the chain. In the diagram, pentan-2-ol has become pentan-3-ol. Many aromatic isomers exist because substituents can be positioned on different parts of the benzene ring. Only one isomer of phenol or hydroxybenzene exists but cresol or methylphenol has three isomers where the additional methyl group can be placed on three different positions on the ring. Xylenol has one hydroxyl group and two methyl groups and a total of 6 isomers exist.
metamerism--------------
This type of isomerism is due to the unequal distribution of carbon atoms on either side of a functional group. Metamers belong to the same homologous series, for example diethyl ether and methyl propyl ether.
POSITION ISOMERISM
Isomerism caused by the difference in the position of functional group in the same chain is termed as position isomerism.
Example: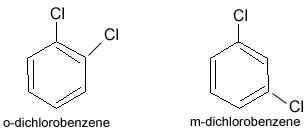
METAMERISM
This type of isomerism is due to the unequal distribution of carbon atoms on either side of functional group. Metamerism belongs to same homologous series. Example: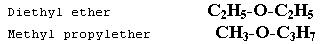
YOUR WELCOME....HOPE YOU DONT NEED AN EVEN BETTER ANSWER!!!LOL
No dude i dont need a better ans..Its the best..I looked fr it in many boooks..Thanks yaar..