Which one among the following is a polar aprotic solvent?
A) DMSO
B) Crown Ether
C) DMG
D) All
E) None
Write in increasing order the values of Heat of Hydrogenation of these compounds. Explain PLZ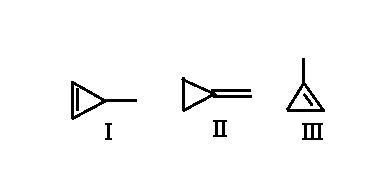
Which one among the following is a polar aprotic solvent?
A) DMSO
B) Crown Ether
C) DMG
D) All
E) None
Visit www.anujkalia.blogspot.com for your daily powerful physics problems. These are neither easy nor too difficult. I bet you'll love them.
P.S.
1)Sorry for (something like) spamming.
2)The blog has been around for an year now. That gives you about a 100 thumping good problems if you scan the archives.
3)Try the blog. I would have.
hydrogenation is an exothermic reaction.
So, more unstable the reactant, higher the heat released (for product remaining same)
Now analyse stability of reactants
here the major cause of unstability is ring strain or angle strain
sp2 carbons prefer a bond angle of 120° and sp3 carbons prefer 109.5° whereas those in the reactants (in the ring) have angle of 60° so, among the carbons present in ring sp3 is more stable than sp2
I am considering only carbons in the ring
in I
2 sp2 and 1 sp3
in II
2 sp3 and 1 sp2
in III
2 sp2 and 1 sp3
so II is most stable of all (most sp3) so its heat of hydrogenation is least
now in I and III no. of sp2 and sp3 carbons in ring is same
so we next consider the stability of the alkene. (ie of the double bond)
As double bond of III is more substituted, it is more stable than I
Hence stability order is II > III > I
hence heat of hydrogenation order is II < III < I