from manmay
::ORGANIC::
there are points in learning where u want to tell everyone wat all new concepts u learnt...so here is our thread...a thread specially for nawshikhio like me in organic...experts are prohibited except to correct if we are wrong in our concepts...
everyone can post newly learnt concepts which he/she really cherishes to know...
this is strictly organic thread!
repitition of concepts are welcome
hoping this thread will provide great revision to all
cheers!!!
-
UP 0 DOWN 0 0 9

9 Answers
this is the first concept in organic chemistry i really loved!! [1]
prerequisites::
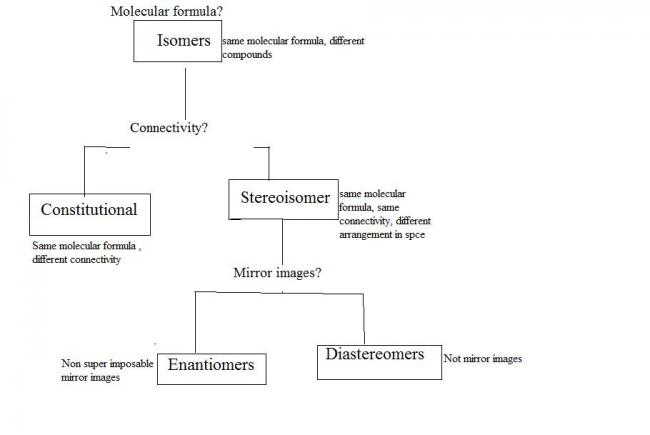
racemic mixture: a 50-50% mixture of two enantiomers...as a result though both enantiomers are individually optically active, the racemic modification is optically inactive!!
enantiomers are equivalent in all physical properties except the angle through which they rotate plane polarised light!! if one is dextrorotary the other is laevorotary!! thus their mixtures cannot be separated by any physical means..
diastereomers are stereo isomers but not mirror images of each other...as a result they have different physical properties and their mixtures can be physically separated!!
resolution: separation of racemic modification to enantiomers...[1]
majority types of resolution depend upon the reaction of organic bases with organic acids to yeild organic salts!!
let for examle we need to resolve the racemic modification of an acid (±)-HA
using an optically active alkaly like substance known as alkaloids extracted from plants... let the BASE = (-)-B
on reaction with the acid, the base forms two salts..
SALT-1:[(-)-BH+ + (+)-A-]
SALT-2:[(-)-BH+ + (-)-A-]
SALT-1 and SALT-2 are:-
a) non superimposable because the acid parts are not superimposable. thus these are stereo isomers.
b) not mirror images as the base parts are not mirror images. thus they are not enantiomers.
they are stereoisomers but nit enantiomers. so they are diastereomers...diastereomers differ in physical properties and thus are separated..
now the separated salts are treated with acid (H+) to give the respective enantiomers of the racemic acid taken and we get the alkaloid back as a salt...[1]
bases used: i) (-)-brucine ii) (-)-quinine iii) (-)-strychnine iv) (+)-chinchonone
acids used: like (-)-malic acid..
to resolve alcohols is tricky since they are neither appreciably acidic nor basic..so, we attach an acid HANDLE to the alcohol to form salt by reacting with bases, and when no more required the handles are removed...
BASIC PRINCIPLE:: A racemic modification is converted by an optically active reagent into a mixture of diastereomers which can then be separated..[1][1]
i hope this is complete....additions and editions are welcome...[1][1]
Calculation of Specific rotation:
Specific rotation at given temperature and wavelength = (measured rotation)/(Sample pathlength in decimeters * concentration in grams/mL of solution)
Maximum number of stereoisomers ≤ 2^n , where n= number of stereocentres
I also found this table helpful:
http://www.targetiit.com/iit-jee-forum/posts/organic-thread-15446.html
general confusion....................
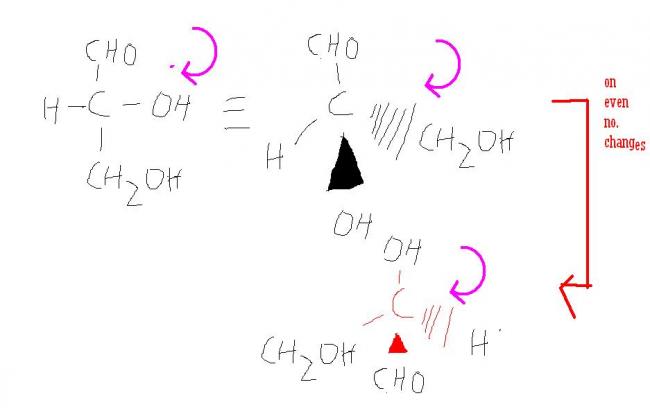
actually i had confusion in converting into these projections ...thats why i gave it here
wat was the above post supposed to mean? it gives the same compound alligned in different manner in space!!![7][7][7]
about E2
An E2 reaction is regioselective. The major product is the
more stable alkene, unless the reactants are sterically hindered
or the leaving group is poor. The more stable alkene is generally (but not always) the more substituted alkene. The more substituted alkene is predicted by Zaitsev’s rule: It is
the alkene formed when a proton is removed from the
β -carbon that is bonded to the fewest hydrogens.
An E2 reaction is stereoselective: If the β -carbon has two
hydrogens, both E and Z products will be formed, but the
one with the bulkiest groups on opposite sides of the double
bond is more stable and will be formed in greater yield. Anti
elimination is favored in an E2 reaction. If the -carbon has
only one hydrogen, only one alkene is formed, since there is
only one conformer in which the groups to be eliminated are
anti. If the reactant is a cyclic compound, the two groups to
be eliminated must be trans to one another; in the case of
six-membered rings, both groups must be in axial positions.
Elimination is more rapid when H and X are diaxial in the
more stable conformer.
about E1
An E1 reaction is regioselective. The major product is
the most stable alkene, which is generally the most substituted
alkene. An E1 reaction is stereoselective. The major
product is the alkene with the bulkiest groups on opposite
sides of the double bond. The carbocation formed in the
first step can undergo both syn and anti elimination; therefore,
the two groups to be eliminated in a cyclic compound
do not have to be trans or both in axial positions. Alkyl substitution
increases the stability of a carbocation and decreases
the stability of a carbanion.
The Effect of the Solvent on Nucleophilicity
Why, in a protic solvent, is the smallest atom the poorest nucleophile even though it is
the strongest base? How does a protic solvent make strong bases less nucleophilic?
When a negatively charged species is placed in a protic solvent, the ion becomes solvated
. Protic solvents are hydrogen bond donors. The solvent molecules
arrange themselves so that their partially positively charged hydrogens point toward
the negatively charged species. The interaction between the ion and the dipole of the
protic solvent is called an ion–dipole interaction. Because the solvent shields the nucleophile,
at least one of the ion–dipole interactions must be broken before the nucleophile
can participate in an SN2 reaction. Weak bases interact weakly with protic
solvents, whereas strong bases interact more strongly because they are better at sharing
their electrons. It is easier, therefore, to break the ion–dipole interactions between an iodide ion (a weak base) and the solvent than between a fluoride ion (a stronger
base) and the solvent. As a result, iodide ion is a better nucleophile than fluoride ion in
a protic solvent