 49
49i use the following mechanism...
frst i take tertbutyl alcohol and mix it with conc HCl and shake it...resulting in tertbutyl chloride
next i take methanol and treat it with HCl in presence of ZnCl2 to give methyl chloride.
next i couple tertbutyl chloride and methyl chloride by the use of the foll mech..
R-X → R-Li → (R)2CuLi → R-R'
(Li) (CuX) (R'-X)
when R=1°, 2° or 3°
and R'=1°
so resulting compound is neopentane...next we do free radical substitution to get neopentyl chloride
any shorter method??
 39
39Oxidise neopentyl alcohol to neopentyl aldehyde by P.C.C.
Reduce the aldehyde to neopentane by Wolff-Kishner or Clemmensen reduction.
Now use free radical substitution.
But we ought to avoid free radical substitution...there must be a cleaner method. Will get back to you on this.
 39
39Of course...there is another way!!!
We need to use a strong aprotic solvent(which obviously favours SN2 regardless of bulkiness of beta carbon). My first thought was DMSO, but it is not as strong. I googled and found an interesting abstract about hexamethylphosphoric triamide -
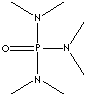
"Hexamethylphosphoric Triamide is a (high boiling point) strong aprotic solvent which does not contain an O-H bond but contains a bond that has a large bond dipole."
The abstract stated that primary alkyl chlorides could be synthesized by reacting the primary alcohol with conc. HCl in HPTM solvent(which is also resistant to acid attacks. This is because, as you can see in the structure, all nitrogens are tertiary and hindered, and the P=O bond is not as polar as the C=O bond).
The result of 2-octanol and neopentyl alcohol took a little longer(50 - 75 minutes), but the final product "was uncontaminated and clean of rearrangement products".
This method of using a strong aprotic solvent works because the alpha carbon is primary which is the necessary requirement for a good yield of SN2 product(but it is not sufficient as beta carbon can be bulky, which comes into play in solvents other than HPTM/DMSO/etc)
 1
1u may use SO2Cl2 wer the carbocation is not formed
 39
39Yaar in that case PCl3 and PCl5 are best used...I hadn't thought of that..lol.
Both will require to be reacted in presence of PYRIDINE to ensure SN2-type reaction, or carbocation does form!
