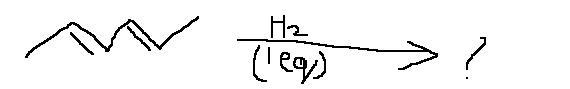this looks like hydrogenation of alkenes.. since u have used only 1 eq of h2 , hydrogenation will take place only at on of the two double bonds....
14 Answers
hydrogenation ????without any catalyst at RT???
seems something else to me ....i havent studied yet but dis question seems to involve sum funda of conjugated dienes ...
i completely agree with u ..... maybe i have fallen for some trick... :)
Eure wat's the answer...
don't ask me for the reason. I just read it somewhere or just an illusion. But the product should be a ring: cyclohexane. I think this should be the answer. i"m still searching for reason
Diels Alder reaction toh nahi hai, there is no dienophile. So doesn't seem like a chance for cyclization...not sure about this but even I think only one double bond will be reduced.
common guys so easy it wud be a 1,4 addition of H & double bond wud b in middle
yeah...thats wat i said b4 ...it has to be something abt conjugated dienes ....
see there wil be resonance in that molecule ....and one of the canonical forms will be an allylic carbocation ..and there will be 1,4 addition.....i diont know much .... ask some one who has studied that chapter ....he will help u best ... or refer any standard book
well that catalyst thing was obvious..
dhruvin is rite[6]
and yeah after i read in my book abt name (1,4 addition)..then reaction became obvious..but at first sight it was new ...tahts why posted[1]
Also,
KCP = 1,2 addition since it occurs fast.
TCP = 1,4 addition since resonance is basically pi-bond shifting, which takes time but stabilises the intermediate.