Plzzz reply
Consider confirmational analysis of butane about C2-C3 bond.It is believed teh predominant conformers about C2-C3 bond are Anti and Gauche while rest contribute negligibly (to equilibrium mixture)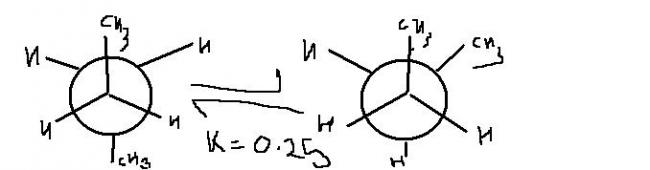
Q1 Ignoring the presence of other isomers ,% of anti at 27°C is??
Q2If temp incresed to 127°C,% of gauche form is doubled .Calculate entahlpy change.
Q3 If temp increased further ,comment on % of Anti and gauche
-
UP 0 DOWN 0 2 1
