hmm.. he is using mCPBA, epoxiding agent of course, and solvent is CH2CL2 which will prevent the clevage of epoxide to glycol. so.. will give oxirane at double bond's place... Then I read about LDA somewhere (proabably at Pritish's post) that it is Lithium diisopropyl amide and is very strong base... so it will look after very acidic H.
Found it intresting ..so posting here...
not at all my doubt...
idnitfy A and B
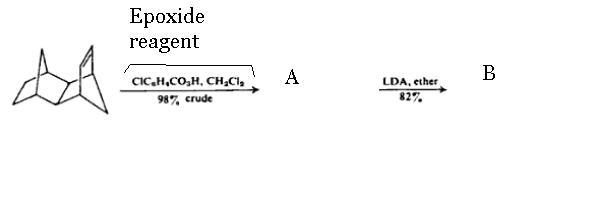
-
UP 0 DOWN 0 2 10

10 Answers
What's the formula of the epoxide reagent? Can't see clearly..
hmm.. let me think.. a bit confused regarding abstraction of H.. wait..
This is what Pritish did with that question: 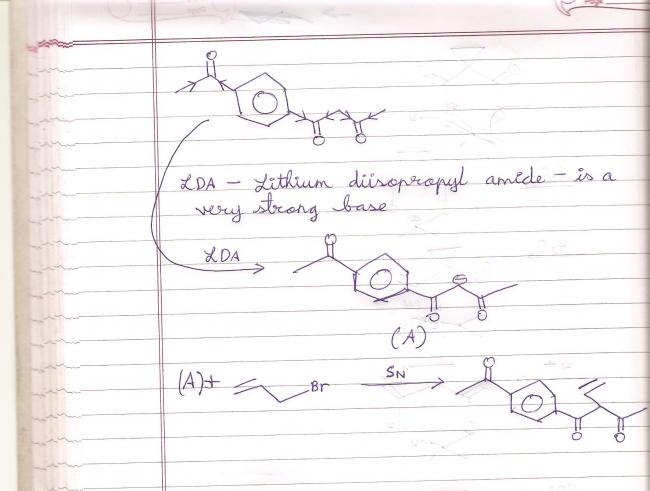
So LDA will abstract the most acidic H, and where would we get that? Carbons adjacent to oxygen seem a good bet, since oxygen is so electronegative....
There are no leaving groups on the epoxide substrate, I initially thought LDA would work on the epoxidizing agent-turned-carboxylic acid as chlorine is a leaving group there. I wonder if the carbanion will react on the epoxide itself?
I want to see the final answer...its a bit tricky..thats why this ques is here..