Answers given are:
1. bc
2. bc
3. c
4. a
Paragraph (Questions may have multiple answers)
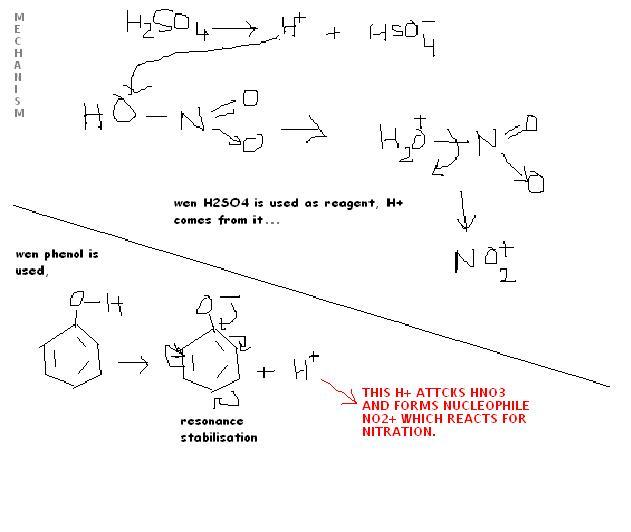
Nitration reaction is classified under electrophilic substitution where the electrophile is NO2+. The reagent used in this reaction is conc. HNO3 + conc. H2SO4.
Q1. What is the role of conc. H2SO4 in this reaction?
(a) It is a good dehydrating agent so, it can remove water to form the corresponding nitro- compound
(b) In presence of it, HNO3 behaves as a base and can give OH- ion to form the electrophile NO2+
(c) conc. H2SO4, by giving H+ facilitates the formation of electrophile
(d) In its presence, the solvent becomes polar and the reaction is faster
Q2. A student A tries to nitrate phenol by taking phenol and conc. HNO3. Another student B performs the same reaction by taking phenol, conc. HNO3 + conc. H2SO4.
Which of the following is/are correct?
(a) The student A will not get any product (if he gets any it will be very less)
(b) The student B gets two products
(c) The student A gets two products
(d) None of these
Q3. Referring to the above question, which of the following is/are correct?
(a) The rate of reaction for student A is negligible
(b) The rate of reaction for student A is less than but comparable to that for B
(c) The rate of reaction for student A is more than that for student B
(d) None of these
Q4. A third student C takes the following: phenol, conc. HNO3 and heats the mixture.
(a) He obtains only one compound
(b) He doesnt obtain any product
(c) He gets two products
(d) Heating will cause explosion (added this instead of none of these [3])
-
UP 0 DOWN 0 0 13

13 Answers
st.b reacts phenl wth conc.H2SO4 n nitric cid .it involves the formation of electrophile first then addition rkn. while direct rkn in the other
direct reaction: how will it occur without electrophile.
it is due to conc. sulphuric acid that electrophile is formed. refer sir's notes
my ans are:
1) b c
2) a b
3) a
4) a
[its surprising y 3 ka c hai... i wud really want to know the reason if that option is correct..]
Q2. bc
he will get two products o-nitrophenol and p-nitrophenol
Q3. explanation for rate:
the rate determining step for electrophilic substitution reaction is the attack of the π-electron cloud to the nucleophile.
So, more the charge density on π-electron cloud, faster is the rate
As -O- is a better ERG than -OH (+M effect -O- has complete negative charge), so, the rate of reaction for case I will be more