and the reason given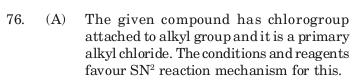
cholorogroup attached to alkyl group??How
it is attached to benzylic grp..
acc. to NCERT it shud go by SN1......how can NCERT be wrong??
20 Answers
Elimination kyun? It's not like its a bulky nucleophile or something...
Here it will be SN2. It is the same reason why allylic substrates prefer SN2 to SN1. In SN2, reaction occurs by means of transition state, in which CHARGES are not full but PARTIAL. Transition state is also stabilised by resonance. Here the nucleophile is also such that bimolecular mechanism will be preferred, unhindered, direct and to the point. Note that partial charges are more stable than full fledged charges, so SN2 intermediate is more stable than SN1 intermediate in such cases.
Hence I second Asish on that count.
govind...organic chemistry favours one thing. One damn thing. And it is called stability.
Khud sochke dekho...which is more stable in this case? A fully charged intermediate, or a partially charged transition state? NCERT is good for the basics, but sometimes we have to think on our own...
haha I was expecting that type of ans in the solutions :P
Bhai even if the answer key had been wrong, I would've believed myself because I gave an explanation. No worries :P
ans given is SN2
www.unifiedcouncil.com/key.aspx
@pritish
dude u were right for this one!
@ Ishan...wait till the 16th for official answers to come out or carry on this debate of SUBSTITUTION VS ELIMINATION
so wat's the official answer??
wait till the 16th for official answers to come out or carry on this debate of SUBSTITUTION VS ELIMINATION
@pritish
dude a man of 143 kg is lazy enough to move but powerful enough to get the job done hope u can draw the analogy here.....
(a) SN2 (as strong base and primary halide)
elimination is not possible
Elimination cannot occur, Double Bond kahaan banaaoge ?!
Even i think SN2, but govind's is a valid reason.
E1 elimination will take place
substitution will not take place because the size of nucleophile is large enough to prevent it
govind... recheck nucleophilic substitution in aromatic systems..
allyl and benzylic grps behave as simple alkyl grps
read any standard chem buk (solomons or morrison) for this effect
in this case elimination is not possible..
but the substitution will take place through SN1 mechanism...see the stability of carbocation
E1 reaction is favoured in compounds in which the leaving grp is at a secondary or tertiary posn.
SN reactn is favoured where leaving grp is at primary posn....

