please give your replies
1) Ph=N=C=O ----(1. CH3MgBr)----> ----(2. H2O------>
2) Ph--Br ----(Mg)---> A --(1. C*H3COCH3)---(2.H+)---(3.heat)---> B
B------(NBS)---->C-----(AgNO3/H2O)---->D
D-----(O3/H2O, Zn)---------> E + F
Identify A to F
( Note that C* is C14 )
3) Why is diethyl ether not used as solvent for rxn. involving RLi unlike in
RMgBr reactions?
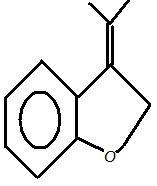
4) 
-
UP 0 DOWN 0 0 9

9 Answers
4. You make Br go out because the +ve there is 3 degree + allylic, so put OH there. And since this OH is not allylic, so there should not be any problem with it.
(You might think that why didn't I relocate the double bond. Think?? )
3.) The reason can be attributed to the fact that Li has a very small size and hence a very high amount of energy is liberated on solvation of Li to Li (aq).
The energy liberated plus high amount of electro positive character enable the R- to slowly bring about an elimination reaction of the diethyl ether:
R:Li + H-CH2-CH2-OCH2CH3 ----> RH + CH2 CH2 + Li+ -OCH2CH3
CH2 + Li+ -OCH2CH3
These are however much more stable in hydrocarbon solvents.
In case of RMgX, the mentioned effects are very minute to bring about such effects.
1.) I presume CO will come out.. Try writing the reactions.
2. Firstly, Ph- will attack at carbonyl carbon and then hydrolysis will yield an alcohol.
NBS should probably replace OH by Br. (I'm not sure cause I tried and failed) Since there can be no oxidation of -OH and also no benzylic position empty for substitution.. Wait for some replies.
first Br- will leave.
conjugation with double bond will take place.
positive charge on terminal carbon attacks on lone pairs of oxygen.
then finally hydrogen leaves to provide stability.