wouldnt beta be the lease stable because the free radical is located on the double bond carbon? if not, then your response to this thread, and the atgs stuck in basics thread dont match
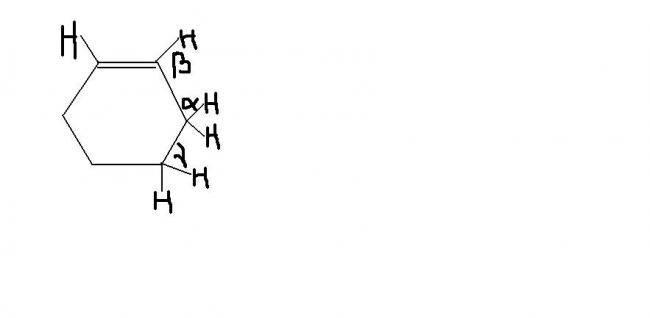
arrange BE of (C-H) bonds indicated by α, β and γ....
-
UP 0 DOWN 0 0 20

20 Answers
Subho I have no idea!! i just know it. I'm looking it up right now in organic chem textbook. Wait
@madhumita...why is free radical attached with a double bond unstable???please reply yaar..
agreed BDE of β>γ>α
i misinterpreted the question the hyperconjugation or say resonance as madhu.. says will make α H open to substitution (allylic). agreed with vector, pranav p also
@madhumita...
u said...''''Well gamma is not adjacent to any double bond, but it is more stable than Beta. coz Beta is a radical ON the double bonded carbon itself, which is unstable.''''
q is y is it so???
yes tat is the right answer. Thats what Vector and I said. Read our explanations
The basic thing is simple: You want the free radical to be on a single bonded carbon, and this single bond should be adjacent to a double bond. In this case, therefore, alpha is most stable. Then u have gamma. Well gamma is not adjacent to any double bond, but it is more stable than Beta. coz Beta is a radical ON the double bonded carbon itself, which is unstable.
huh....what is now this hyperconjugation thing/??ok let me study more theory!!
the more i study organic the more i realise i know nothing...[2][2][2]
after much trying to understand, my answer is the order of BE will be reverse of order of stability of free radical at that point,,,
so ,
free radical stability is in order α>γ>β
so BE is in order α<γ<β..
i think i am correct,,,
Look at your "stuck in basics" post..in tat, I is more stable than III...same logic
α<γ coz α has conjugation whilst γ does not
so α free radical must be stabler so bond energy α<γ
now this is against ur answer manmay...
and what is expl for β??????